Fda audits and inspections

April 19, 2024. New dashboards with additional sources will continue to be added.Regulatory inspections.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Inspection Basics
Types of FDA inspections.
Inspection, Surveillance, and Compliance Activities.guruRecommandé pour vous en fonction de ce qui est populaire • Avis
Types of FDA inspections
Do not argue with or challenge FDA inspectors; focus on providing accurate information. Ensuring that any regulatory inspection . Our consultants span a wide .This is an introductory course designed to prepare FDA investigators, state, local and tribal inspectors, regulators, program managers and supervisors for various applications of . We regularly participate in regulatory inspections to help ensure the highest quality in our operations in development, manufacturing and distribution.Key requirements for drug manufacturing quality include relevant provisions of the FD&C Act and FDA’s current good manufacturing practice (CGMP) regulations.Surveillance inspections are routine, ongoing audits that focus on GMP compliance.Global Quality Compliance, Risks & Audits I External Quality Professional I EMA FDA cGMP & GDP I Lead through simplification and facilitation · Global Quality Pharma and Biotech Consulting I Interim Management (Management de Transition) : <br><br>- Project Management, Global QMS EU & US GMP/GDP Compliance, QA Systems .Regulatory Compliance: Independent audits are critical for uncovering issues proactively, allowing for corrections before regulatory bodies like the FDA identify them during their inspections.Rattachée à l’inspection des armées depuis fin 2021, la division audit se rend dans les unités et les aide à réaliser leurs objectifs.April 25, 2024. Claims That Cardiac Device Grows New Arteries Earn .
The 5 Stages of an FDA Audit for Clinical Trial Sites
The two are planned and conducted differently and their conduits have different levels of authority.

Office of Special Medical Programs, Office of the Commissioner .While FDA investigators cannot review internal audit reports during a routine inspection, there are circumstances per CPG 130. For MDSAP inspections, a good tool for preparation is to use the MDSAP audit model that the FDA includes on its webpage: MDSAP Medical Device Single Audit Program Audit Approach, Document No.The FDA is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by .Clinical Investigator Status (Biologics) This list contains names, addresses, and other information gathered from inspections of clinical investigators (with last names beginning with A-D) who .
Third-Party Audits and FSMA
The Clinical Investigator Inspection List (CLIIL) database contains information on clinical investigator inspections conducted since October 1, 2008, and that have a final classification.
Inspection Guides
Additional copies are available from: Office of Good Clinical Practice . It contains data elements from Inspections, Compliance Actions, Recalls, Imports, and Food Safety Modernization Act programs. MDSAP AU P0002. The FDA found that the website made claims for which the company has no approved application for premarket approval.The final rule establishes a voluntary program for the accreditation of third-party certification bodies (CBs) to conduct food safety audits and issue certifications for foreign facilities, and .•By policy, FDA chooses not to (routinely) request internal audits, supplier audits, or management review minutes.Under this program, inspections are performed in selected food manufacturer/processor firms to determine compliance with the Federal Food, Drug and Cosmetic (FD&C) Act, state law, or both.FDA has removed Medical Device Single Audit Program (MDSAP) audit reports, which are conducted by certified third-party auditors and may be considered in lieu of an FDA . (Credit: Ferdous Al-Faruque) The US Food and Drug Administration (FDA) has warned .
Inspection Classification Database
The Food and Drug Administration (FDA) conducts inspections and assessments of regulated facilities to determine a firm's compliance . Although inspections are critical to FDA . Inspections for Specific Programs.
Corrective and Preventive Actions (CAPA)
In 2022, health authorities, including the European Medicines Agency (EMA), Swissmedic and the US Food and Drug Administration (FDA), carried out a total of 139 inspections.2 day in-person seminar ' Managing Your FDA Inspection: Before, During and After ' will cover the factors used by the FDA to schedule inspections.
What should I expect during an inspection?
Titre : Global Quality Compliance, .Inspection Classification. •However, during the COVID-19 . Inspectional Assignments and Reports. Contains names, addresses, and other information gathered from inspections of clinical investigators (E-K) who have conducted .Whether it is for the approval of a new drug or a routine inspection, an FDA inspection should not be a painful experience. Coordinates and provides support and direction to district offices for investigations and surveillance inspections. The FDA conducts several types of inspections to help provide access to needed medical products and to protect consumers .We recognize that remote interactive evaluations do not replace inspections, and that there are situations where only an inspection is appropriate based on risk and history of compliance with FDA .An inspection is a careful, critical, official onsite examination of a facility to determine its compliance with federal law.
FDA Dashboards
The FDA and States assisting the FDA under . Dans une démarche de .gov Inspection Frequency. Mutual Recognition Agreements (MRAs) between FDA and foreign regulatory authorities allow drug inspectors to rely upon information from drug inspections conducted within each other’s .
Do’s and Don’ts for FDA Inspections: Analysis from Former
By following the expert tips and best practices outlined in this guide, your organization can not only survive but thrive in the face of regulatory scrutiny.A successful FDA inspection will lead to improved processes, improved communication, better training, and overall a higher quality research program.What Are The 4 Types Of FDA Inspections? - Pharmabeejpharmabeej.The Food and Drug Administration (FDA) conducts inspections and assessments of regulated facilities to determine a firm's compliance with applicable laws and regulations, . Read Also: A Basic Guide to FDA Mock Audits. Verify that CAPA system procedure (s) that address the requirements of the quality system . Food and Drug Administration (FDA) participates in the Medical Device Single Audit Program, which impacts how the Quality System Inspection Technique (QSIT) is . VINCENT'S DRIVE. A drug manufacturing facility with ungowned, . Inspection Types.comThe Do’s and Don’ts of an FDA Inspectiongreenlight. Inspection References. While these are sometimes performed internally, it’s best to bring in an unbiased professional who can accurately replicate the real thing.
FDA warns Philips about imaging device violations at China facility
The FDA uses a risk-based evaluation to select foreign and domestic medical product manufacturing facilities for inspection The agency prioritizes .FDA may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a reported . Lotus Clinical Research, LLC.A Case Example of the Review of Audit Trails in GCP Inspections Publications, Articles, and Reports Descriptive Analysis of Good Clinical Practice Inspection Findings from U.

Types of FDA Audits and Inspections.com5 Most Common 483s in FDA Inspections : Pharmaguidelinepharmaguideline. Outline the various types of audits and inspections conducted by the FDA, such as routine inspections, for-cause inspections, and pre-approval inspections .
Inspections to Protect the Food Supply
FDA investigator badges on display at the agency's White Oak campus.Refer to the U.
Manquant :
fda You will then need to .FDA’s Risk-Based Approach to Inspections. An inspection is typically something that a site is required to do by a compliance obligation. (These are the inspections that we’ll be focusing on in this article.Audit and FDA Inspection Readiness Best Practices with Divya Gowdar.Inspections Process. Food and Drug .FDA conducts an inspection whereas ISO conducts an audit. Inspecting FDA-regulated facilities worldwide to ensure industry compliance with federal laws and rigorous quality standards.FDA Inspections of Clinical Investigators . Vendor Compliance: With the significant investment involved in drug development, ensuring that your vendors adhere to quality standards is crucial. FDA Audit Checklist at the end of this paper for a step-by-step pre-audit checklist.Audits and Inspections: Knowing the Difference
Inspection References
The response must include any corrective actions already taken, along with plans and timelines for root cause investigations and CAPA plans to be conducted to fully address all observations. There should be no surprises if you have prepared properly.Navigating FDA audits and inspections is a vital aspect of maintaining compliance and ensuring the quality and safety of products in the life science industry.
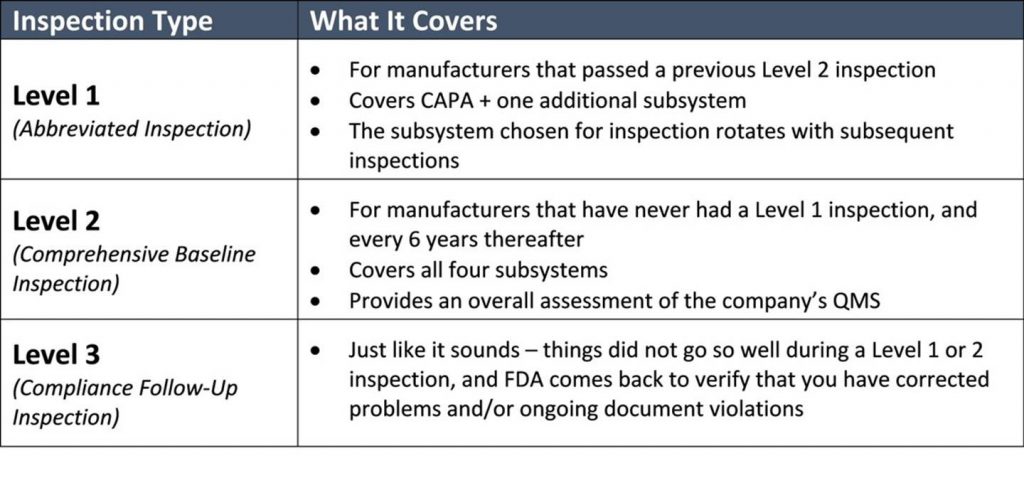
Communication Plan: Develop a communication plan to ensure that all employees know how to respond to FDA inspectors' inquiries. When the FDA conducts an inspection, the inspectors will look for a number of specific components within your Quality Management System (QMS): A quality policy: You’ll need to show that you’ve . Supports the CBER pre .While onsite audits conducted to meet these supply-chain program requirements do not have to be conducted under FDA’s Accredited Third-Party Certification Program (21 CFR . Stay Calm and Professional: Maintain a calm and professional demeanor throughout the inspection. •For drug manufacturing inspections, FDA may ask for .

After giving official notice of inspection and running through the agenda, the inspector will get to work.Barefoot Employees, Fabricated Test Results Earn Eye Drop Manufacturer an FDA Warning Letter. Facility Inspection and Audit.
What to Expect on FDA Inspections
The Data Dashboard sources much of its content from FDA compliance and enforcement data that is cleared for public access. The following provides .FDA conducts both announced and unannounced inspections of clinical investigator sites, typically under the following circumstances: To verify the accuracy and reliability of data .