Fda cleared products

Differences: FDA-Cleared and FDA-Approved Medical Devices
14 Best LED Face Masks, According to Dermatologists and
This exclusive energy output allows for more precise applications and reduced healing time.Medical Devices Cleared or Approved by FDA in 2021. Free shipping* 4.A 510(K) is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a .Devices@FDA is a catalog of cleared and approved medical device information from fda. Of these, 84% (or 1,477 devices) were eliminated . For each product, you can find information about what the device is, how it . Browse and shop our diverse collection of products that have been cleared by the FDA.A breakdown of the CAD types across the 104 FDA-cleared products is shown in Fig. This is done to protect the public from potential .The US Food and Drug Administration’s internal database of 510 (k)-cleared medical devices was searched for additively manufactured devices cleared .The FDA cleared for marketing the first over-the-counter (OTC) antigen test for COVID-19. CADt is the most common product type, representing 59% of products, followed by CADe with 19%.With several at-home devices being FDA cleared, it's easy and safe to do red light therapy at home.FDA-cleared sharps disposal containers are available in a variety of sizes, including smaller travel sizes to use while away from home. The first AI algorithm was cleared by the FDA in 1995, and fewer than 50 algorithms were approved over the next 18 years.Subnovii is FDA-cleared, and the first plasma device that uses LF+ technology: low-frequency + a patented wavelength and power combination.Temps de Lecture Estimé: 5 min
Recently-Approved Devices
Food and Drug Administration This number is listed on each FDA 510K Clearance Letter with the indicated status of this number on the FDA website: PART 878 — GENERAL AND PLASTIC SURGERY DEVICES. ACON Laboratories’ Flowflex COVID-19 Antigen Home Test, originally authorized for emergency use in 2021 .
Technology
Section 510 (k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical . Showing 1–16 of 28 results.Smoking cessation products approved or cleared by the FDA are shown to help people quit smoking and can even double your chance of quitting successfully. The more complex a product is and the higher its potential risk for human or animal use, the more ., the FDA must approve or clear the device. Moreover, Eneo enhances the anti-aging treatment by use of micro vibration therapy*, gold* and detox blue light. Before a medical device can be . Subnovii is not your ordinary plasma pen. it includes links to the device summary information, manufacturer, .“FDA approved!” Maybe you saw those words on a company’s website or in a commercial promoting a product or treatment.
Medical Device Databases
An FDA 510 (k) cleared medical device is allowed to make claims on the product that have been reviewed and cleared by the FDA.
iRESTORE Laser Hair Growth System
The FDA can hand out clearances or approvals for all sorts of things, from certain food products and additives in cosmetics (although they do not approve .What the FDA does approve is specific products: food additives, drugs, certain medical devices, etc.Before a medical device can be sold or marketed in the U.How to know if a medical device is FDA-approved, cleared, or authorized. The FDA regulates the sale of medical devices and monitors the safety of all regulated medical products. She instructs us to avoid harsh or over-exfoliating products while using LED; rather, reach for . This article will review the FDA approval and clearance process for medical devices so you can understand the different terms.Products that pass this clearance process may be referred to as “FDA cleared” or “FDA listed,” but this is not the same as “FDA approved,” which only relates .These Eko products are cleared by the FDA for sale in the United States: Eko CORE 500™ Digital Stethoscope FDA Clearance Statement K230111. Eko CORE™ FDA Clearance Statement K200776 Eko DUO™ FDA Clearance Statement K170874.See the complete list of FDA-cleared algorithms here.SaMD Cleared by the FDA: The Ultimate Running List. It also prevents unsubstantiated (false) claims from appearing on labeling or advertising media (3).This list, created by Orthogonal and Brian Binkowski, contains information on 562 individual Software as a Medical Devices (SaMD) cleared by the FDA. The FDA does not test the product themselves, but instead reviews the data that has been submitted.
Learn if a Medical Device Has Been Cleared by FDA for Marketing
What Does The FDA Regulate?
510(k) Clearances
Products that pass this clearance process may be referred to as “FDA cleared” or “FDA listed,” but this is not the same as “FDA approved,” which only relates to the prescription drugs . Subpart E – Surgical Devices.
Are There FDA Registered or FDA Certified Medical Devices?
Required for: most Class II devices with a predicate. A list of products and companies with FDA-cleared sharps . Gaining FDA clearance typically has the shortest timeframe out of the two – usually within 90 days of submission – primarily because it doesn’t involve as rigorous testing as . We carry mobility products of a variety of styles and brands including EWheels Medical, EV Rider, Shoprider, Merits, Afikim and others. Examples of cosmetics are perfumes, makeup, moisturizers, shampoos, hair dyes, face and body cleansers, and shaving preparations.Devices@FDA is a catalog of cleared and approved medical device information from FDA. Class II devices include infusion pumps used to give intravenous (IV) . We synergistically combined the most advanced technologies for skin rejuvenation: targeted red light, infrared light and thermal treatment.The technology behind Eneo anti-aging.Specs: FDA-cleared, four light wavelengths: red (630 - 700nm), blue (400 - 470nm), infrared . Compare Devices Shop Anniversary Sale COMPARE DEVICES Watch how it works.The data used to create this list was pulled from publicly available sources such archived reports from the FDA and Basil System’s regulatory search tools.Browse through more than 200 FDA-cleared products created by more than 100 manufacturers to find algorithms that best support your patients and workflows. The products in each list contain . On April 18, 2024, the Food and Drug Administration approved alectinib (Alecensa, Genentech, Inc.The Regulation Number for Aspen Laser Products as General & Plastic Surgery Devices is 21 CF 878.March 18, 2024.
FDA Cleared Vs FDA Approved: What’s The Difference?
The FDA or Food & Drug Administration is a US federal agency responsible for protecting public health by ensuring the safety, efficacy, and security of drugs, biological products, medical devices, food .The products listed here include some of the newest medical technology available.So when we encounter products claiming to be FDA approved or FDA cleared, it becomes essential to understand the fundamental difference between them.
FDA Cleared Medical Products
Medical devices range from simple tongue depressors and hospital gowns to complex . The products listed in this section include some of the newest medical technology from the year 2021. Free shipping in the Contiguous U.
FDA Cleared Vs FDA Approved: What’s The Difference?
With this milestone, the company .6 | 25,896 Reviews.FDA Cleared vs Approved.) for adjuvant treatment following tumor resection in patients . Subnovii’s best-in-class plasma technology is the result of over 6 .Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized hydrogen peroxide, and other sterilization methods (for . market were cleared between 2019 to 2022––more than . (57) Lux Collection dpl IIa – LED Treatment Panel Anti-Aging & Acne. This list, created by Orthogonal, Brian Binkowski and . Food and Drug . The FDA does NOT issue registration certificates. See visible regrowth in just 3 months. AS SEEN IN VOGUE, INSTYLE, AND HEALTH!

Grow thicker, healthier hair back naturally with our FDA-cleared products. Braftovi (encorafenib . Both FDA Clearance and FDA Approval indicate that the product is considered safe and effective for distribution on the U.

However, the numbers have increased rapidly in the past decade, and more than half of algorithms on the U. As this list is a work in progress, we .
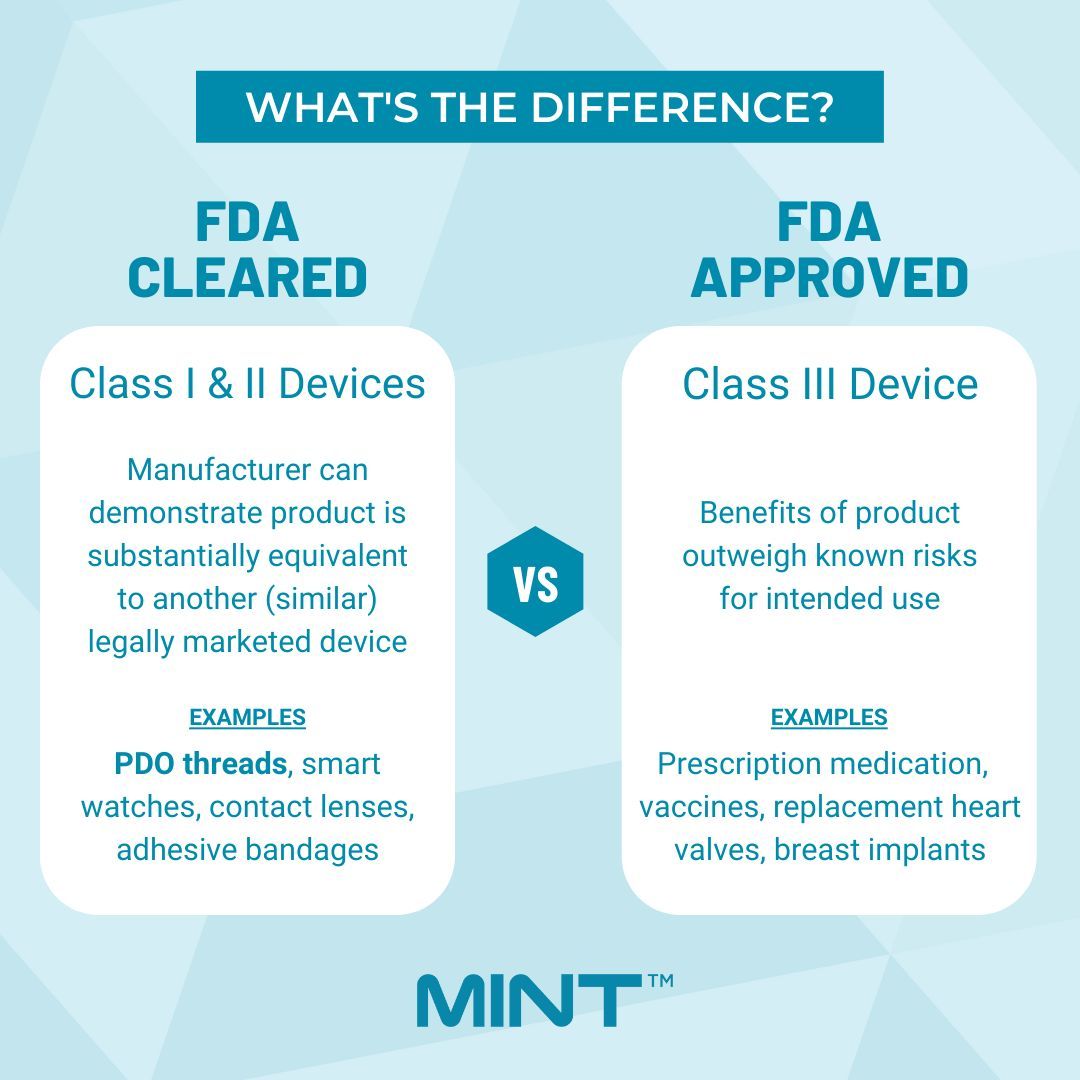
FDA approves products based on the relative risk they pose to consumers. FDA clearance is granted to Class II medical devices that can prove .The FDA does not approve cosmetics.FDA Cleared Products | reVive Light Therapy® | Vio® | dpl®.

As illustrated .We carry FDA cleared medical products meet and exceed all standards for your piece of mind and requirements set by the FDA.
510(k) Premarket Notification
It's a non-invasive, painless treatment, but it's still very important to read directions .Class II: These medical devices have a moderate risk to consumers and must demonstrate that they are “substantially equivalent” to similar products that have already received FDA clearance.gov510(k) Devices Cleared in 2023 | FDA - U. FDA Approved means that the FDA, in its own words, .Products from foods to medicines to health monitoring applications tout phrases like “FDA Registered,” “FDA Approved,” and “FDA Cleared. All of our power wheelchairs and mobility scooters are FDA cleared.510(K) Premarket Notification - Food and Drug Administrationaccessdata. Some marketers may say their products .

Eko CORE 500™ Digital Stethoscope FDA Clearance Statement K233609.And some medical devices must be FDA cleared or FDA approved before being marketed and sold in the U.Lux Collection Lip Care – LED Lip Plumping.