Fda deciding when to submit 510 k
This step-by-step guide to preparing your 510 (k) submission aims to put the requirements in easy-to-understand terms and includes some helpful, actionable and .This document supersedes FDA’s guidance Deciding When to Submit a 510 (k) for a Change to an Existing Device (K97-1), issued on January 10, 1997.
Recently we have added one Contraindication to our IFU of a previously 510(k) cleared device. The FDA guidance Deciding When to Submit a 510(k) for a Change to an Existing Device suggest (in the Figure 2, flowchart A, Point A2) to submit a change being effected (CBE) 510(k).NWX-FDA OC Moderator: Irene Aihie 11-16-17/1:00 pm E T Page 1 .Deciding When to Submit a 510 (k) for a Change to an Existing Device: Guidance for Industry and Food and Drug Administration Staff. This number is commonly referred to as the 510(k .Deciding When to Submit a 510 (k) for a Software Change to an Existing Device (Software Modifications guidance) These guidances clearly define key terms relevant to device .” you have to . Effective Date January 13, 2005 .Starting October 1, 2023, all 510 (k) submissions, unless exempted (as described in Section VI. This protocol implements the recommendations provided in the FDA guidance document “ Deciding When to Submit a 510(k) for a Software Change to an Existing Device: Guidance for Industry and FDA Staff. ” De Novo Summary (DEN180001) Page 4 of 13• Reporting changes in the 510(k) Outline www.Recently, the U.This comment supports FDA’s recommendation for evaluation of simultaneous changes (ref.Temps de Lecture Estimé: 10 min
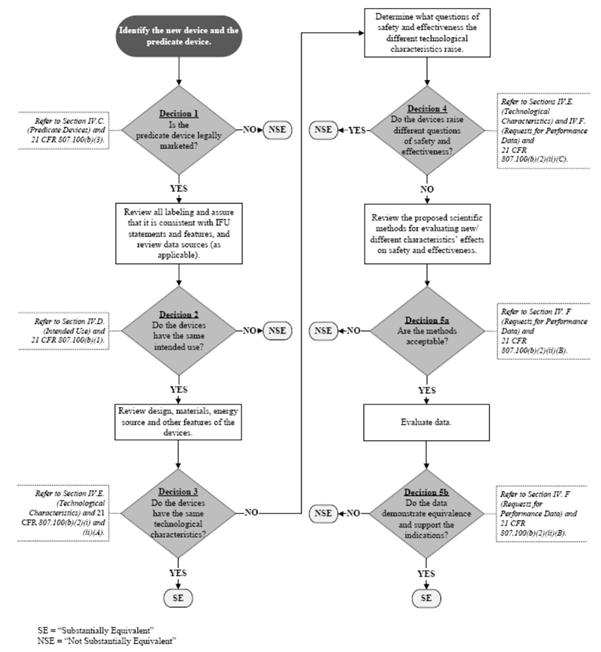
Is a new 510(k) required for a modification to the device?
Per the FDA Guidance, The Special 510(k) Program, a Special 510(k) is appropriate when it is a change to the manufacturer’s own device and . Device (K97-1) on January 10, 1997 to provide guidance on this regulatory language. Everything you need to know about the FDA 510 (k) submission. This document is intended to provide guidance in the preparation of a regulatory submission. I'm now in process to put together the documents for a 510k and I am not sure, if we need a Traditional or a Special 510(k) application.A 510(k) Summary or 510(k) Statement must be included in your 510(k) submission in order for FDA to begin its scientific review of the 510(k) submission.the guidance document, Deciding When to Submit a 510(k) for a Change to an Existing Device, is one of these actions. Issued by: Food . This guidance is not intended to implement significant policy changes to FDA’s current thinking on when submission of a new 510(k) is required for a software change to a 510(k)-cleared device (or group of . This is a critical go-to document for the average Regulatory Engineer, so I have been thinking about what I would change to make it more straight . FDA Webinar: Final Guidances on Deciding When to Submit a 510(k) for a Change to an
Deciding When to Submit a 510(k)
Some of these scenarios include: When evaluation of the change(s) to the device generally involves greater than three scientific disciplines (e.The Food and Drug Administration (FDA or Agency) is announcing the availability of the guidance entitled ``Deciding When to Submit a 510(k) for a Change to . This guidance will assist industry and Agency staff in determining when a software (including firmware) .
510(k) Submission Process
Deciding When to Submit a 510(k) for a Software Change to an Existing Device .gov; 3 • Deciding When to Submit a 510(k) for a Change to an Existing Device • FDA CDRH LEARN • Guidance include guiding principles for evaluating changes • Main types of changes: – Labeling – Technology, Engineering, or performance – Materials • Evaluate risk profile FDA’s Guidance . History Written/Revised Approved Allentities move and nothing remains still,这个世界上唯一不变的是改变。对于医疗器械法规从业者来说,可能最烦恼的一个词语就是“改变”。大部分国家/地区的法规对于医疗器械的变更的总是那几个形容词:实质性的,影响安全性和有效性 .On October 25, 2017, FDA issued a final guidance: Deciding When to Submit a 510 (k) for a Change to an Existing Device.To address some confusion surrounding the guidance Deciding When to Submit a New 510 (k) for a Change to an Existing Device, the FDA has worked diligently to enhance predictability, consistency and transparency while maintaining a “least burdensome approach. What is an FDA 510 (k) submission - and how do we complete one? It's a .变更遵循原则: 2017年10月25日,FDA正式发布了Guidance “Deciding When to Submit a 510(k) for a Change to an Existing Device, 按照此指南决定对现有设备进行更改时是否需要提交新的510(k),应遵循以下指导原则: This guidance is a final version of .A of the final guidance, Electronic Submission Template for Medical Device 510 (k) .The 2017 guidance specifies ten guiding principles that manufacturers should consider when deciding whether to submit a new 510 (k) or to document changes made in .

not intended to provide guidance on when a new 510(k) is required for changes to an existing device.
510(k) Premarket Notification
The draft of this document was issued on August 8, 2016.Temps de Lecture Estimé: 1 min FDA Webinar: Final Guidances on Deciding When to Submit a 510(k) for a Change to an
 3.png)
The new updated guidance provides greater detail of the regulatory .Hi, I'm new here and need your help.Date Received: 08/03/2023: Decision Date: 04/18/2024: Decision: Substantially Equivalent (SESE) Regulation Medical Specialty: Dental 510k Review PanelDeciding when to submit a 510 (k) for a change to an existing device: guidance for Industry and Food and Drug Administration staff - Digital Collections - National Library .
December 21, 2023
Additional information about changes that may require a new premarket notification are provided in the FDA guidance documents entitled Deciding When to Submit a 510(k) for a Change to an Existing Device 90-91 : Add scope of wording to include an existing device, or family of devices: “.

Center for Devices and Radiological Health.However, after searching for a while, I have found .

Deciding When to Submit a 510(k) for a Change to an Existing Device .firmware) change to a medical device may require a manufacturer to submit and obtain FDA clearance of a new premarket notification (510(k)).
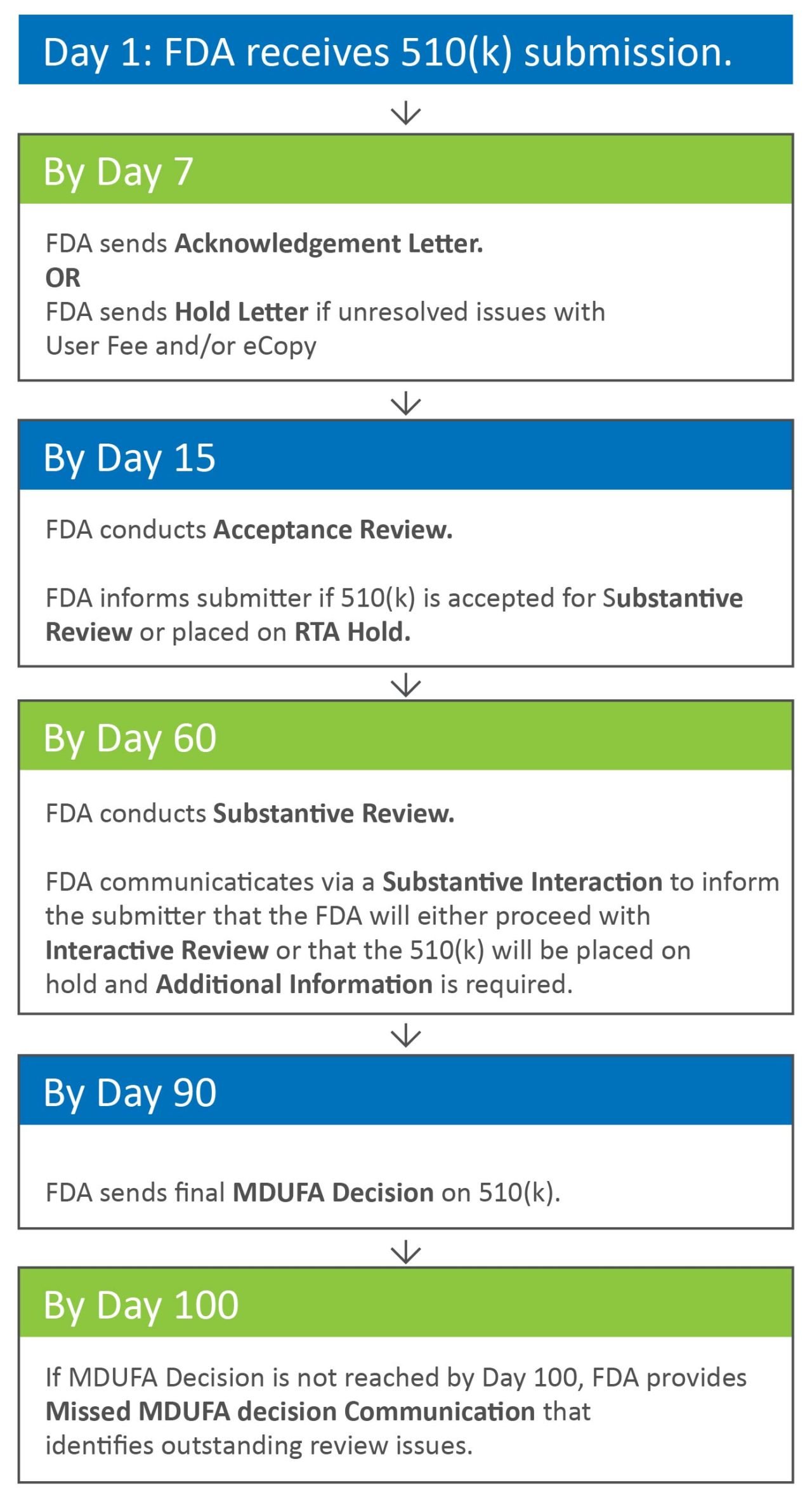
Premarket Notification 510 (k) submissions for medical devices are reviewed and processed by the Center for Devices and Radiological Health (CDRH) within the Food .内容提示: Contains Nonbinding Recommendations Deciding When to Submit a 510(k) for a Change to an Existing Device Guidance for Industry and Food and Drug Administration Staff Document issued on October 25, 2017.

Content current . Requirements to Consider: FDA Guidance Document: Deciding When to Submit a 510(k) for a Change to an Existing Device found at: .Page 13 of the Special 510(k) guidance document also provides a number of scenarios under which the FDA believes it’s not appropriate to submit a Special 510(k).Deciding When to Submit a 510(k) for a Change to an Existing Device.Guidance Issuing Office.
DE NOVO CLASSIFICATION R IDX-DR
The guidance covers changes .when to submit a 510(k) for a change to an existing device. This protocol implements the recommendations provided in the FDA guidance document “ .Deciding When to Submit a 510(k) for a Change to an Existing Device, January 10, 1997 . This guidance is not intended to implement significant policy changes to FDA’s current thinking on when submission of a new 510 (k) is required. Rather, the intent of this guidance is to enhance .1The guidance is far reaching and will apply to all device manufacturers who intend to make changes to their devices going forward.Additional information about changes that may require a new premarket notification are provided in the FDA guidance documents entitled Deciding When to Submit a 510(k) . Section 510 (k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical . Food and Drug Administration (FDA) issued final guidance on deciding when to submit a new 510(k) premarket notification application for a change to an existing medical device.On October 25, 2017, the Food and Drug Administration (FDA) issued a final guidance document, Deciding When to Submit a 510(k) for a Change to an Existing Device, which describes when a change in a medical device would require the submission of a new premarket notification (commonly referred to as a 510(k)). Office of Device Evaluation Document Issued On: January 10, 1997.Following the FDA Guidance, Deciding When to Submit a 510(k) for a Change to an Existing Device, a Change Being Effected (CBE) 510k is appropriate when .因市场需求带来的产品的设计变更,对于出口美国的医疗器械而言,是否需要重新申请一个新的510(k),是基于美国食品药品监督管理局(FDA)发布的指导原则:Deciding When to Submit a510(k) for a Change to an Existing Device。 何时需要重新提交510(K)? The recommendations in this draft guidance document .
510(k) Clearances
for a 510(k) premarket notification submission before commercial introduction.