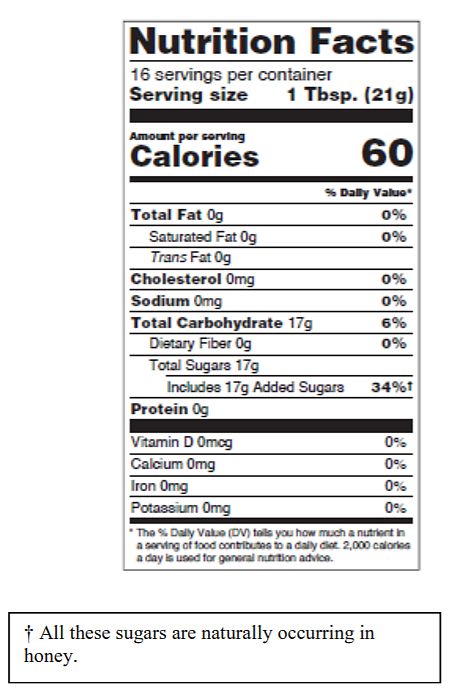
Fda declaration of added sugars

Added sugars are either added during the processing of foods, or are packaged as such, and include sugars (free, mono and disaccharides), sugars from .
FREQUENTLY ASKED: FDA LABELING REQUIREMENTS FOR “ADDED SUGARS”
A similar desire was behind the transition in the first place.
FDA Issues Final Guidance on Declaration of Added Sugars for Single Ingredient Products and Certain Cranberry Products.Compliance Date, Added Sugars, and Declaration of Quantitative Amounts of Vitamins and Minerals: Guidance for Industry .This guidance also discusses labeling of added sugars, as well as formatting for lines (e.Contains Nonbinding Recommendations .April 17, 2019.Temps de Lecture Estimé: 8 min
CFR
foodqualityandsafety. The Declaration of Added Sugars on Honey, Maple Syrup, Other Single-Ingredient Sugars and Syrups, and Certain Cranberry Products: GuidanceThe FDA plans to soon release additional guidance to help manufacturers in complying with new labeling requirements, including a guidance to address the declaration of added sugars on packages and containers of honey, maple syrup and certain cranberry products.5, addressing the voluntary declaration of “added sugars” as part of the additional written nutrition information under 21 CFR 101. June 28, 2019 By Riëtte van Laack .from the declaration of “Total Carbohydrate,” “Total Sugars,” and “Added Sugars” on the Nutrition Facts label ( Docket Number FDA-2015-P-1201) (Ref. Food and Drug Administration is reviewing comments on its draft guidance to inform its approach to the declaration of added . 05 Jun 2019 --- Fruit purees may be a viable solution for US food companies rethinking their product formulation due to the soon-to-be implemented US Food and Drug Administration (FDA) rules on the declaration of “added sugars” on food .By the fall of 2027, added sugars in school meals would be limited to no more than 10% of the total calories per week for breakfasts and lunches, in addition to limites .The FDA might find that addressing added sugar is feasible without impinging on the concern that total sugar labeling inappropriately captures sweetened food it considers healthy. 2015-2020 Dietary Guidelines recommendation that no more than 10 percent of calories be .FDA issues a final guidance to provide clarity on the labeling of added sugars for single-ingredient packages or containers of pure honey, maple syrup, and other single . In the meantime, the agency has addressed the unique properties of allulose in the draft . April 13, 2018.On November 6, 2023, FDA hosted a virtual public meeting on strategies to reduce added sugar consumption in the U. The added sugars declarations, together with the other nutrient declaration on the . This could take the form of requiring manufacturers to disclose added sugar . Yet, potential health impacts and cost-effectiveness of this policy .Constituent Update.
Guidance for Industry: NFL Questions and Answers
Food and Drug Administration (FDA), “Added sugars include sugars that are added during the processing of foods .” The draft guidance, when finalized, will advise food manufacturers of our intent to exercise enforcement .Finally, FDA does not require the declaration of added sugars for products that contain less than 1 gram of added sugars per serving, so long as no claims are .5g), but says it is “also proposing .Some specific examples of FDA’s definition of added sugars include: agave nectar. thickness of lines) and leading (e.The Food and Drug Administration (FDA) has announced that by January 1, 2020, the Nutrition Facts label will display added sugars information in grams per serving and .115(g)(5)), to ensure that FDA considers your comment on this draft guidance before we begin work on the final version of the .In 2016, FDA mandated the labeling of added sugar content on all packaged foods and beverages. Food and Drug Administration ( FDA) published the final guidance on the Declaration of Added Sugars on Honey, Maple .Although you can comment on any guidance at any time (see 21 CFR 10. The agency also noted its development of draft guidance .

Additional copies are available from: Office of Nutrition and Food Labeling . For most Americans, the main sources of added sugars are sugar .
Making Sense of Added Sugars Labeling [INFOGRAPHIC]
Updated 5:31 AM PDT, April 24, 2024. Print Bulletin PDF.By the fall of 2027, added sugars in school meals would be limited to no more than 10 percent of the total calories per week for breakfasts and lunches, in addition to . Food and Drug Administration is extending the comment period by 45 days on the draft guidance for industry about the declaration of added sugars on . After all, it's a great way to make packaged foods more transparent to consumers., fulfilling FDA’s mandate under the White House National Strategy on Hunger, Nutrition, and Health to “assess [] the evidence base” for, and hold a public meeting regarding, “future steps the federal government could take . Finally, FDA does not require the declaration of added sugars for products that contain less than 1 gram of added sugars per serving, so long as no claims are made about sweeteners (caloric or non-caloric), sugars, added sugars, or sugar alcohol content.The added sugars declaration is just one requirement in two comprehensive new food labeling rules, which issue changes to daily values, serving sizes, and more. Regarding single-ingredient packages or containers of pure honey, maple syrup and other single ingredient sugars and syrups, . FDA Provides Flexibility in Calculating Added SugarsHealth educators lauded the decision to add a line for added sugars on the new label.

Food and Drug Administration issued a draft guidance today to provide its current view on the declaration of carbohydrates, total sugars and added sugars for products that .On June 18, 2019, the Food and Drug Administration (FDA) has published The Declaration of Added Sugars on Honey, Maple Syrup, Other Single Ingredient Sugars and Syrups and Certain Cranberry Products: Guidance for Industry. In 2016, FDA issued a final rule updating the Nutrition .The FDA is advising manufacturers that it intends to exercise enforcement discretion regarding the requirement that allulose be included in the amount of total sugars and added sugars declared on . brown rice syrup.What are added sugars? According to the U.This guidance is intended to identify for manufacturers specific, additional isolated or synthetic non-digestible carbohydrates that we intend to propose adding to the list of those that meet ourThe first draft guidance answers questions related to compliance, labeling of added sugars, rounding as it relates to the declaration of quantitative amounts of vitamins and minerals, and label .4 Food; designation of ingredients.
Comment Period Extension on Guidance on Declaration of Added Sugars

11(b)(2)(ii)(A) and the voluntary use of menu labeling requirements on online third-party food ordering platforms.On average, Americans consume more than 13% of total calories (or almost 270 calories) per day from added sugars, with intakes particularly high among children, adolescents, . To address added sugar, the FDA could first require its disclosure on the nutrition facts panel.

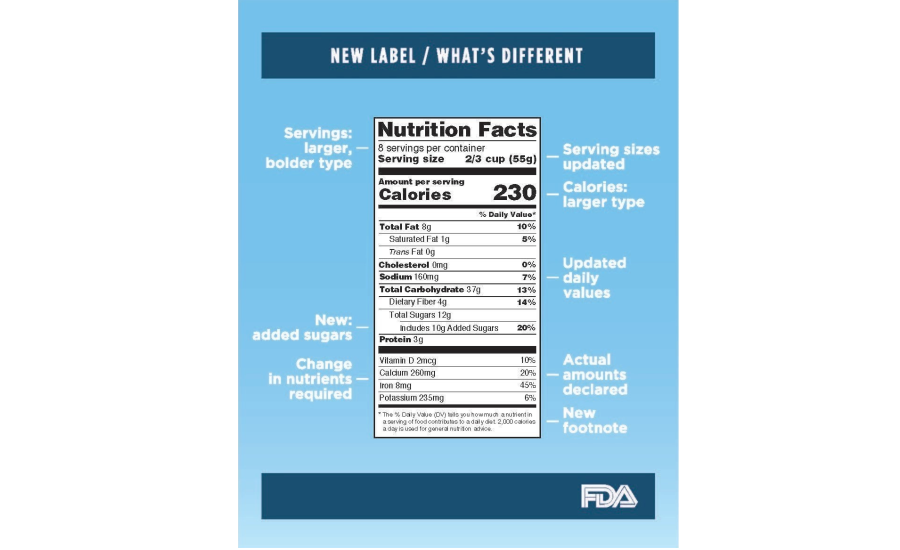
Interactive Nutrition Facts Label
food’s formulation.Guidance to provide clarification on the labeling requirements for single-ingredient packages of pure honey, pure maple syrup, and other pure sugars and syrups, which are not .The FDA’s decision gives producers of honey, pure maple syrup, agave syrup and other single-ingredient sugars and syrups what they wanted since most objected to listing added sugars when.FDA’s definition encompasses the types of added sugars that were evaluated in the science underlying the .The bitter truth about added sugar - Harvard Healthhealth. 14 of the draft guidance: Here is the text below for your .The Reagan-Udall Foundation will host a public meeting on front-of-package labeling on November 16, 2023.The new US FDA regulations on added sugar label declarations will be implemented starting January 1, 2020.

Food and Drug Administration (FDA) is issuing a final guidance to provide clarification on the added sugars labeling requirements on packages and/or containers .The Food and Drug Administration (FDA or we) is announcing the availability of a draft guidance for industry entitled “The Declaration of Added Sugars on Honey, Maple Syrup, and Certain Cranberry Products: Guidance for Industry.The Declaration of Added Sugars on Honey, Maple Syrup, and Certain Cranberry Products; Guidance for Industry In the Federal Register of March 2, 2018 (83 FR 8953), we announced the availability of . Statement on new guidance for the declaration of added sugars on food labels for single-ingredient sugars and syrups and certain cranberry products.eduFDA Issues Final Guidance Concerning Added Sugars - .
Added Sugars on the Nutrition Facts Label
FDA clarified that divulging the amount of added sugar in a standard menu item can help consumers meet current dietary recommendations and stay within caloric .







