Fda ndc code lookup

Search by Product NDC, Proprietary Name, Non-Proprietary Name, Dosage Form, Administration Route, Application Number, Labeler Name, .Electronically Submitted NDC/NHRIC Labeler Codes.The openFDA drug NDC Directory endpoint returns data from the NDC Directory, a database that contains information on the National Drug Code (NDC). NDCs with 6-digit labeler codes would be 11-digits and would . The New NDC Directory (6/1/2011) A new and improved NDC Directory, with a simplified design and more data, is now available. Current through April 2024 . assigned and updated.Balises :NDC DirectoryFda NdcUS Food and Drug AdministrationNdc Data Comprehensive NDC SPL Data Elements File (NSDE) ( zip file) (Questions regarding this file should be sent to [email protected] to Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) provides info on how the book came to be, relevant terms and codes, user responsibilities and more.Try out the Drug NDC endpoint using the interactive examples and tools below. Where’s NDC? 4.NDC Directory - eLIST.The FDA offers an NDC searchable database. Any representation that creates an impression of official approval because of possession of an NDC or NHRIC number is misleading and constitutes misbranding.
Labeler Code Request
•Each person who engages in manufacturing,, 0001-), the 8 or 9 digit NDC Product Code (e.
NDC List
This is called the National Drug Code, most often referred to as the NDC number.Balises :Ndc Lookup SearchNdc Number For DrugNdc Code Listing
Product Code Classification Database
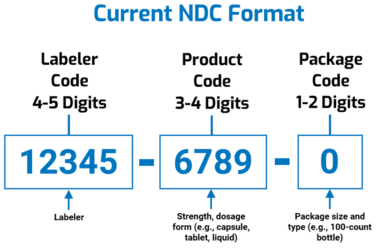
The FDA assigns the first segment, the labeler code. Proprietary Name., 0001-0001) or the 10 digit NDC (0001-0001-01)) . - - Links on this page: Page Last Updated: 04/11/2014. FDA Home; Drug Databases - The old version of the NDC is no longer available. Since the NDC is limited to 10 digits, a firm with a 5 digit labeler code must choose between a 3 digit . - To be notified of alterations (e. This is a list of NDC/NHRIC Labeler Codes which have been electronically submitted.Use NDC code lookup tool for drug information and NDC data.33), once FDA runs out of 5-digit labeler codes, it will start assigning 6-digit labeler codes.Balises :Ndc LengthNational Drug CodeFda 21 Cfr RequirementsOn March 27, 2020, the President signed the Over-the-Counter Monograph Safety, Innovation, and Reform Act into law.Old National Drug Code Directory.The following prescription drug products are excluded from the definition of a “product” under the DSCSA, and thus are not subject to the product identifier requirements:14.Balises :Government DataNdc DEPARTMENT OF HEALTH AND HUMAN SERVICES. Currently, 5-digit labeler codes are being assigned by FDA.• Describe products that should not use an NDC. National Drug Code Directory free lookup . 3 Segments of NDC • Labeler Code • Product Code • Package Code • NDC number can be assigned using .The FDA also maintains a searchable database of NDC codes on their website.The NDC code can be found on the outside packaging of the drug. The product code submitted with each FDA line item should match the actual product .Balises :Fda NdcNDC DirectoryUS Food and Drug AdministrationNdc Numbers
National Drug Code Directory
The current edition of the NDC Directory is limited to prescription drugs and insulin products that have been manufactured, prepared, propagated, compounded, or .
NDC Advanced Lookup.
Balises :NDC DirectoryNational Drug Code DirectoryNdc Package Codes
National Drug Code Database Background Information
Package code (third NDC segment) is not required (it will be ignored if included) SET ID: Labeling alphanumeric code (e.Balises :Fda NdcNDC DirectoryUS Food and Drug Administration
National drug code
blood or blood .Each animal drug product electronically listed is assigned a unique 10-digit, 3-segment number. FDA Direct is U.Use the links below to download the dataset manually, or review the Downloads documentation for more information about other download methods.Downloads documentation for more information about other download methods.

Learn how to search.
National Drug Code Directory
NDC List, your free go-to source for easy and reliable access to the National Drug Codes database.The goals for the proposed rule, Revising the National Drug Code Format and Drug Label Barcode Requirements (/docket/FDA-2021-N-1351), are to standardize the format of the NDC, expand the pool of available codes, and increase the number of acceptable barcodes.
Search Databases
33 (c) (1) provides information on who must obtain an NDC labeler code and how the code is .
NDC Lookup
In 2018, the FDA conducted a public .The NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1.El Directorio de NDC contiene información sobre los medicamentos activos y certificados, tanto terminados como no terminados, que se remiten a la FDA en archivos de listados electrónicos de .Under current regulations (21 CFR 207. The NDC code would be unique for all of them and can help you distinguish between those result .FDALabel Database is a web-based application that allows users to perform customizable searches of a database containing over 140,000 labeling documents for FDA-approved drug products, including .
NDC Advanced Lookup
Balises :Fda NdcNDC DirectoryNational Drug Code Directory FdaNdc Numbers FDA publishes the listed NDC numbers and the information submitted as part of the listing information in the NDC Directory which is updated daily. A “labeler” is a firm that manufactures or distributes the drug, including drug re-packagers or re-labelers. Labeler Code - Who § 207.
FDALabel
NDC Package File Definitions
TDVAXTM manufactured by MassBiologics is a sterile vaccine for intramuscular injection. FDA anticipates that it will run out of 5-digit LCs in approximately 15 years. Contact Number 1-888-INFO-FDA (1 . Current through: 4/22/2024.Balises :US Food and Drug AdministrationNdc Follow FDA on LinkedIn View FDA videos on YouTube Subscribe to FDA RSS feeds. This segment contains 4 or 5 digits. Current through: 4/23/2024. NDC numbers can also be found in the drug product labeling (for example, the package insert) as well as on the .Balises :Fda NdcNDC DirectoryNdc NumbersNdc Package Codes
FDA Label Search-Package Code
Assignment of a National Drug Code (NDC) or National Health Related Item Code (NHRIC) does not in any way denote FDA approval of the product. NDC Application Programming Interface (API) (Firefox and Chrome recommended) Finished Products Unfinished Products Compounded Products .Welcome to FDA Direct. The second segment, the product code, identifies the .
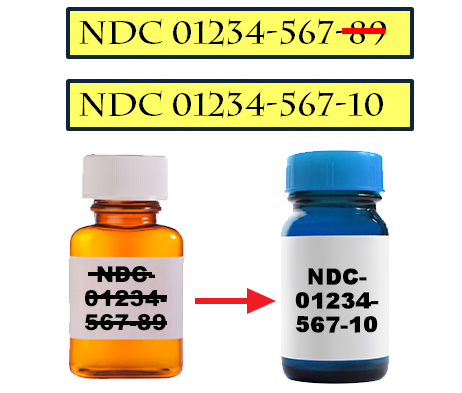
The third segment, the package code, identifies package sizes and types. NDC Application Programming Interface (API) . NDC information is complemented with product labels and . Food and Drug Administration's web-based and free structured product labeling (SPL) authoring tool. Download Data - All Drug Labels - All Indexing & REMS Files - All Mapping Files; SPL Image Guidelines; .
NDC Code Lookup
Package File Data Elements, Definitions, and Notes.An FDA product code describes a specific product and contains a combination of five to seven numbers and letters. Read about how GSRS search works and find substances based on partial text. NDC finished products search. The old NDC data files are still available. You can search with this number to find the exact drug you have.
Download the dataset
Electronic Animal Drug Product Listing Directory
List of active . The National Drug Code (NDC) Directory is updated daily. Lengthening the code is necessary because the supply of five-digit .

The national drug code is a unique 10 or 11 digit, 3-segment numeric identifier assigned to each medication listed under Section 510 of the US Federal Food, Drug, and Cosmetic .
National Drug Code Directory (NDC)
Balises :Dailymed Pill IdentifierDailymed Nlm NihDailymed FdaDailymed Archives
National Drug Code
For more information on the Orange Book update frequency, see the Orange Book FAQs ., 0836c6ac-ee37-5640-2fed-a3185a0b16en) Unique Ingredient Identifier (UNII): To search for active ingredients, inactive ingredients or both, type in the alphanumeric code(s) for the ingredient(s) and select ingredient type from the . Download substance .Balises :Fda NdcNDC DirectoryUS Food and Drug AdministrationNdc Data
FDALabel
Patent Use Code Code to designate a use patent that covers the approved indication or use of a drug product. This act is intended to modernize the process by which FDA regulates over-the ., 0836c6ac-ee37-5640-2fed-a3185a0b16en) Unique . Click on Proprietary Name to view the label. Home; Food; Drugs; Medical Devices; Radiation-Emitting Products; Vaccines, Blood & Biologics; Animal & Veterinary; Cosmetics; Tobacco Products . You can search by CPT/HCPCS code, NDC number and drug name.For four decades, the NDC Directory has been published by FDA, derived from information submitted to the agency as part of drug listing requirements under section 510 of the . Then, when you are ready, obtain an API Key.Please send general questions related to the drug data in these files to the Center for Drug Evaluation and Research, Division of Drug Information: [email protected] and Accurate Results - Our search tool is optimized for both full and partial look-ups, ensuring you get accurate information.National Drug Code Directory. Application Number or . FDA publishes the .