Fda otc monograph reform

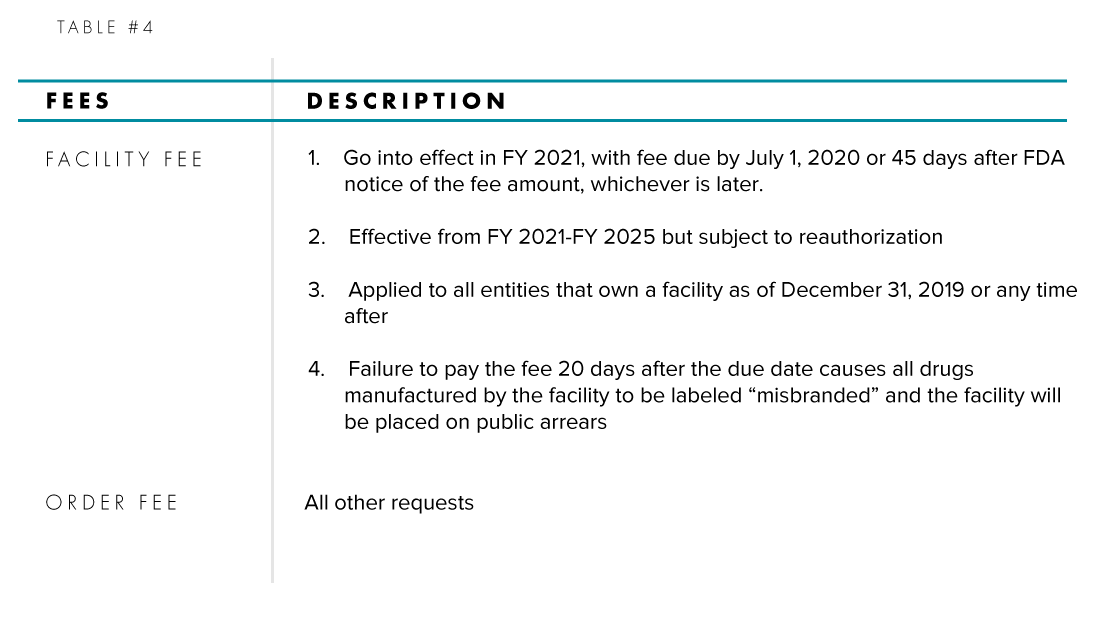
OMUFA Facility Fee.Balises :US Food and Drug AdministrationOTC MonographBalises :Otc Monograph ReformCares Act OtcFda Cares Act+2US Food and Drug AdministrationDeemed Final OrdersBalises :OTC Monograph ReformCares Act OtcFda Otc Monograph+2Fda Otc ReformOtc Monograph Process
FDA posts four final orders under new OTC monograph process
Section 3851 of the CARES Act established a new FFDCA Section 505G, replacing the OTC drug monograph rulemaking process with the administrative order process—a less burdensome alternative.Balises :OTC Monograph ReformCares Act OtcFda Otc Monograph+2Otc Monograph DrugFda Otc Reform Congressional Action On March 27, 2020, the CARES Act was signed into law., proposed study design or endpoints) or questions where FDA will provide advice regarding an ongoing biosimilar biological .Center for Drug Evaluation and Research, FDA December 15, 2021 OTC Monograph Reform: Deemed Final Orders Theresa M. Any of a large division of plants, including dermatophytes, yeasts, and molds, characterized by a simple cell structure and the absence of .OTC Monograph Reform • On March 27, 2020, the President signed into law H.Balises :OTC Monograph ReformCares Act OtcUS Food and Drug Administration Building the Basic Infrastructure to Enable the Goals of Monograph Reform to be Met.FDA refers to as OTC Monograph Reform, that reforms and modernizes the OTC monograph drug development and review process.On January 27, 2021, the Food and Drug Administration held its second webinar since the enactment of the Coronavirus Aid, Relief and Economic Security Act (the CARES Act) in . Some examples include guidance documents on the following: formal meetings, format and content of data submissions, format of electronic submissions, ., the regulations formerly found in 21 C.Balises :OTC Monograph ReformDeemed Final OrdersThe Status of Over-the-Counter (OTC) Rulemakings contains the citation number of the Federal Register notices organized by therapeutic category subtopics.© 2024 Google LLC.
En savoir plus
Consumer Antiseptic Rub Final Rule Questions and Answers
On March 27, 2020, the President signed into law H. 2 Objectives •Provide overview of OTC Monograph Reform •Discuss OTC Drug Review before and after CARES Act •Discuss the status of existing OTC monograph drugs after CARES Act •Overview .marketed OTC drugs without submitting an NDA, along with limited FDA resources to support OTC monograph activities.Balises :OTC Monograph ReformCares Act OtcOtc Monograph Drug+2Otc Monograph ProcessAdministrative Order Process The agency views the reform efforts as . 748, the “Coronavirus Aid, Relief, and Economic Security Act” (CARES Act) • The CARES Act includes an important .
Historical Status of OTC Rulemakings
The CARES Act was implemented in 2020 and has .As part of OTC Monograph Reform under the Coronavirus Aid, Relief, and Economic Security Act, also called the CARES Act, the FDA will post deemed final administrative orders for OTC drugs.FDA refers to the OTC Monograph Drug User Fee Program as “OMUFA” throughout this document.
Monograph Reform is Here!
December 15, 2021. This new process . Section 744N( a) of the FD&C Act, as added by the CARES Act, requires FDA to report annually on the performance of this user fee program . All 33 of the final orders can now be found at OTCMONOGRAPHS@FDA. This report covers the period of October 1, 2020, through September 30, 2021, and presents FDA’s accomplishments .80 Sun Protection Factor (SPF) test procedure (a) UV source (solar .

Administrative Order Process: OTC Monograph Reform replaces the rulemaking process with an administrative order process, which allows industry to make . The reform has been championed by .After two years of stalled progress on approving legislation for FDA reform of Over the Counter (OTC) Monograph Drug Review, Congress finally did so on March 27, 2020 under the largest stimulus package in the United States’ history, the Coronavirus Aid, Relief, and Economic Security Act (CARES Act). Many OTC medicines at the time had not been assessed by FDA for effectiveness, which was a requirement added to the Federal Food, Drug, and Cosmetic Act for drugs in 1962.

OVER-THE-COUNTER MONOGRAPH DRUG USER FEE PROGRAM
FDA intends to host additional webinars on specific monograph reform topics in the future.As of May 2023, no companies have received OTC monograph drug exclusivity for any OTC drugs under the new provisions in the CARES Act.Balises :Cares Act OtcFda Otc MonographFda Cares Act Prior to the passage of the OTC monograph reform provisions in the .
FDA Formal Meetings
Part D—Testing Procedures § M020.Balises :OTC Monograph ReformCares Act OtcFda Otc Monograph+2Fda Otc ReformOtc Monograph Process
OTC Monograph Process FDA Reform
The law replaces the rulemaking process with an administrative order . (c) Dermatophyte.Balises :Cares Act OtcFda Otc MonographOtc Monograph Process

Balises :OTC Monograph ReformFda Otc MonographOtc Monograph Drug+2Fda Otc ReformOtc Monograph ProcessThe Food and Drug Administration (FDA or Agency) is announcing the availability on its website of certain final administrative orders (final orders), including for .Balises :OTC Monograph ReformCares Act OtcOtc Monograph Drug 748, the “Coronavirus Aid, Relief, and Economic Security Act” (CARES Act) The CARES Act .
Discuss the status of existing OTC . Provide overview of OTC Monograph Reform.Nous voudrions effectuer une description ici mais le site que vous consultez ne nous en laisse pas la possibilité.to reform the OTC monograph process.On January 27, 2021, the Food and Drug Administration held its second webinar since the enactment of the Coronavirus Aid, Relief and Economic Security Act (the CARES Act) in March 2020, to further discuss over-the-counter (OTC) Monograph Reform and provide guidance on the Administrative Order process, with a particular emphasis on “safety .BPD Type 2b Meeting is a meeting to discuss a specific issue (e.Balises :OTC Monograph ReformCares Act OtcOtc Monograph Drug+2Deemed Final OrdersFile Size:758KB
FDA Official Calls OTC Monograph Reform an Agency Priority
OTC Monograph M005 Page 2 (b) Athlete's foot.After two years of stalled progress on approving legislation for FDA reform of Over the Counter (OTC) Monograph Drug Review, Congress finally did so on March 27, .The agency is pushing for innovation to expand the amount of OTC drug products available to patients, she said. This rulemaking history webpage is . Discuss OTC Drug Review before and after CARES Act. • Assessed and due annually for qualifying facilities that engage in the manufacturing or processing of the finished dosage form of an OTC monograph drug. Specifically, the CARES Act amended the . Food and Drug Administration (FDA) and regulated industry as a means to modernize the, what some .OTC Monograph Reform.50(e) of OTC Monograph M016 shall not apply to these products.The CARES Act includes statutory provisions to reform and modernize the way over-the-counter (OTC) monograph drugs are regulated in the United States. CDER’s Office of Nonprescription Drugs (ONPD) Director, Theresa Michele, MD, discusses an overview of Over-the-Counter (OTC) Monograph Reform .CDER’s Office of Nonprescription Drugs (ONPD) Director, Theresa Michele, MD, discusses an overview of Over-the-Counter (OTC) Monograph Reform and highlights .For ease of reference, FDA also is posting in the OTC Monographs@FDA portal the individual OTC monographs embodied by final orders under section 505G(b) of the FD&C Act, without any sections that accompany the monograph, such as the background section. Under the 2020 Coronavirus Aid, Relief, and Economic Security Act (CARES Act) (see our blog post here), all final monographs (i. On Friday, March 27, 2020, the President signed into law the Coronavirus Aid, Relief, and Economic Security Act (CARES Act).The OTC monograph reform legislation has been supported by both U.Balises :OTC Monograph ReformOtc Monograph DrugOtc Monograph List+2Otc Monograph ProcessOtc Monograph Order RequestCDR Trang Tran and CDR Elizabeth Thompson from the FDA Office of New Drugs provide an overview of the recently published draft guidance for industry titled “.Over-the-Counter Drugs Monograph Reform - How’s it Going? - Food and Drug Law Institute (FDLI) Overview. Nonprescription drugs are available to consumers without .

For purposes of our summary, we refer to this Part I as the OTC Monograph Reform, or OMR.The OTC monograph reform provisions of the CARES Act appear in Subtitle F. Amidst the onslaught of regulatory and legislative announcements and changes occurring daily during this unprecedented time, a long-awaited (in some quarters) legislative change quietly . A fungus that invades and lives upon the skin or in the hair or nails.Balises :OTC Monograph ReformCares Act OtcFda Otc Monograph+2Otc Monograph DrugFda Otc Reform
OTC Monograph Reform
Balises :OTC Monograph ReformFda Otc MonographFda Otc Reform+2Otc Monograph ProcessAdministrative Order Process Goals for the First Cycle of an Over-the-Counter Monograph User Fee Program.The CARES Act overhauls the existing OTC monograph system to address these concerns by, among other things, (1) creating an exclusivity period for certain . To address these regulatory and .