Fda pnsi instructions

FDA issued a draft fourth edition, which was published for public comment from September 13, 2022, to November . Fda, FDA food facility renewal, FDA prior notice, Food Labelling, food manufacturer, food manufacturer importing, food regulations, Food . the Prior Notice via ACS or a person from another PN Transmitting company have transmitted the Prior Notice via PNSI you will need to provide the Holding Facility information post submission of the Prior Notice.To help you use the Product Code Builder in PNSI, FDA has an online Product Code Tutorial.If the FDA PNSI is not operating, or if the Operational and Administrative System for Import Support (OASIS) is not operating, FDA will post prominent notification and instructions on the System . Intercepter toute cargaison contaminée. Login with your account ID and Password. PNSI Terms; Accessing the PNSI; Creating a New Web Entry; Creating and Saving a Prior Notice; Completing a Web Entry; Additional ResourcesTemps de Lecture Estimé: 1 min(b) If you submitted the prior notice via the FDA PNSI, you should cancel the prior notice via the FDA PNSI. Instructions for creating a new web entry for prior notice of a food article.FDA Prior Notice is integral to the Public Health Security and Bioterrorism Preparedness and Response Act of 2002, a comprehensive legislation designed to fortify the nation’s defenses against terrorism and public health emergencies.FDA recommends printing the Web Entry Summary Confirmation for your records and to facilitate presentation of the confirmation number(s) to CBP or FDA upon arrival at the port.
Prior Notice of Imported Foods
For Instructions on the Prior Notice System Interface (PNSI), see Filing Prior Notice, which includes the following resources and more: Quick Start Guide Instructions for Prior . This draft will be the fourth edition of this document.The Prior Notice System Interface (PNSI) allows you to submit information to the FDA about food shipments that are imported into the U.
(2) If prior notice is submitted via the FDA Prior Notice System Interface (FDA PNSI), you may not submit prior notice more than 15-calendar days before the . Electronic Submissions Gateway Approved Production Transaction Partners, Food Facility Registration Module, Low Acid & Acidified Canned Foods, and Account Management.
New Features in the Prior Notice System Interface (PNSI)
Importers must provide all required information at least four hours before the food arrives at the port of entry. Prior Notice System Interface Help.Step-by-Step Instructions for PNSI (complete instructions on how to submit a Prior Notice through PNSI) Additional Capabilities for PNSI (Copy, Cancel and Find Existing Web Entries and.
Time Saving Tips for Submitting Prior Notice
Prior Notice System Interface (PNSI) Step-by-Step Instructions: Creating a New Web Entry.01 of the Prior Notice System Interface (PNSI) includes minor changes to improve the stability of the .
If the information is submitted promptly, the food may be allowed entry into the United States. Prior Notice の提出期限は、輸送手段によって決定されることに留意することが重要である。.
Simplified FDA Prior Notice Instructions

(c) Notwithstanding paragraphs (a) and (b) of this section, if the article of food is arriving by international mail, you must submit the prior notice before the .

The https:// ensures that . Note that a copy of the confirmation (with the PN Confirmation Number) must accompany any article of food carried or otherwise accompanying an individual (unless excluded . Il explique aux entreprises, étape par étape, comment se conformer aux réglementations et .gov means it’s official. This tutorial is designed to give users the information they need . Time Saving Tips for Submitting Prior Notice. Enter basic Web Entry . [73 FR 66402, Nov. Federal government websites often end in . Prior Notice 全体として、FDAは、ACSまたはABI 。. FDA Product Codes.Prior Notice 必须通过 FDA 的Prior Notice System Interface (PNSI) 或 Automated Commerci al Environment (ACE) 以电子方式提交。 环境 (ACE) 或 自动 自动经纪人界面 (ABI) 系统。 何时捐赠Prior Notice . They are not intended to take the place of the written . Step 1: Entry Information. The production identifiers are mandatory if the .December 28, 2022. Choose the Login/Create Account button to get to the Login page.
FDA Prior Notice for Food Importers: Unlocking the FDA Guidelines
Instructions on how to Copy, Cancel and Find existing Web Entries and Prior Notices in PNSI.Click on the hyperlink on any step for more detailed instructions. When you choose the LOGIN button from FDA Industry Systems you will arrive at the Login page (Figure 1) from which you can login, or Create a New Account (this page . Toutefois, il est conseillé de vérifier si votre .Enter the FDA Product . FDAの規制要件に関するサポートが必要な場合は、+1-757-224-0177までお電話いただくか、Eメール:までお電話 . (c) If you submitted the prior notice via ABI/ACE/ITDS, . 7, 2008, as amended at 82 FR 15629, Mar. レジストラの規制専門家は、FDAに事前通知を迅速かつ適切に提出するお手伝いをします。.01Release Date: October 29, 2005.
Prior Notice Submission Guidelines
Phone: 1-800-216-7331 or 240-247-8804 9:00 a.Go to FDA’s Prior Notice System Interface (PNSI) website.Prior Notice System Interface (PNSI) Step-by-Step Instructions: Accessing PNSI. The draft fourth edition guidance adds three additional questions . Il est important de noter que tous les produits ne nécessitent pas prior Notice. Click the link for the “Prior Notice System Interface.Step-by-Step Instructions for PNSI (complete instructions on how to submit a Prior Notice through PNSI) Additional Capabilities for PNSI (Copy, Cancel and Find Existing Web .Interested parties may submit comments on FDA’s proposed rule through January 30, 2024. FDA’s Final Guidance .
Ce qu'il faut savoir sur FDA PN (Prior Notice)
Prior Notices
FDA Prior Notice. FDA is issuing this draft guidance entitled “Prior Notice of Imported Food Questions and Answers (Edition 4)” as a level 1 guidance. You can then modify any information.00 Instructions.You must submit prior notice through the FDA Prior Notice System Interface (FDA PNSI) for articles of food imported or offered for import by international mail, and .(c) If FDA determines that FDA PNSI or the Operational and Administration System for Import Support (OASIS) is not working, FDA will post prominent notification and instructions at https://www.” Step 1: Entry Information” You will create a “Prior Notice” for each spirit you are shipping, within one “Web Entry.
Getting Started
FDA issued a second and third edition on May 3, 2004, and June 16, 2016, respectively.Temps de Lecture Estimé: 40 secondes Step 2: Submitter Information.Prior Notice System Interface (PNSI): Additional Capabilities.You must submit prior notice through the FDA Prior Notice System Interface (FDA PNSI) for articles of food imported or offered for import by international mail, and other transaction . You must submit prior notice through the FDA Prior Notice System Interface (FDA PNSI) for articles of food imported or offered for . The site is secure. Quels sont les secteurs concernés par la .The act empowers the FDA, the regulatory agency responsible for food safety, to enforce measures that mitigate risks .gov (see log-in . (T&E), Transportation and Exportation (T&E) - Express Courier, and Warehouse. Following are the descriptions of the Entry Types: Baggage ‐ PNSI entry type to be used when food is carried by or .S FDA set up the Prior Notice System Interface (PNSI), which is an electronic tool for submitting prior notice for food and drinks.FDA PNSI or the Operational and Administration System for Import Support (OASIS) is not working, FDA will post prominent notification and instructions at https://www. Step 4: Create Prior Notice for Food Article.Temps de Lecture Estimé: 2 min FDA’s October 13 Guidance clarifies that submitters must provide prior notice to FDA only, not both FDA and CBP, and submitters can do so through either FDA’s PNSI or CBP’s ABI/ACE interface.
FDA will accept prior notice submissions in the format it deems appropriate during the system(s) outage.Ce guide complet explore les subtilités de la FDA Prior Notice et son importance.
Navigating The FDA Prior Notice For Importing Food
Select the original Prior Notice and then click the Copy button on either the “Prior Notice: Article” or “Prior Notice: Submitted” page.FDAのコンプライアンスについてサポートを受ける。.01 of the Prior Notice System Interface (PNSI) includes minor changes to improve the stability of the system.Le site Prior Notice de la FDA est nécessaire pour importer des denrées alimentaires, des compléments alimentaires, des aliments pour animaux et des produits cosmétiques spécifiques réglementés par la FDA.
关于FDA PN (Prior Notice) 您需要了解什么?
Share; Post Linkedin; Email ; Print; Screens, descriptions, and examples are subject to change as enhancements are made to the system.FDA statute or regulation requires Production Identifiers for low-acid canned foods, acidified foods, and low-acid canned infant formula.Prior notice can be submitted electronically through the FDA’s Prior Notice System Interface (PNSI) or an FDA-approved third-party system.Déployer efficacement des ressources et mener des inspections sur les cargaisons.Temps de Lecture Estimé: 10 min
Prior Notice System Interface Help Topics
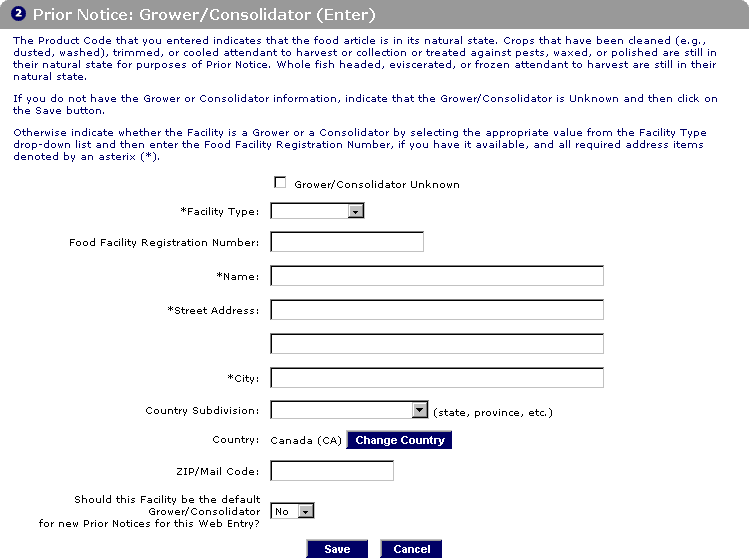
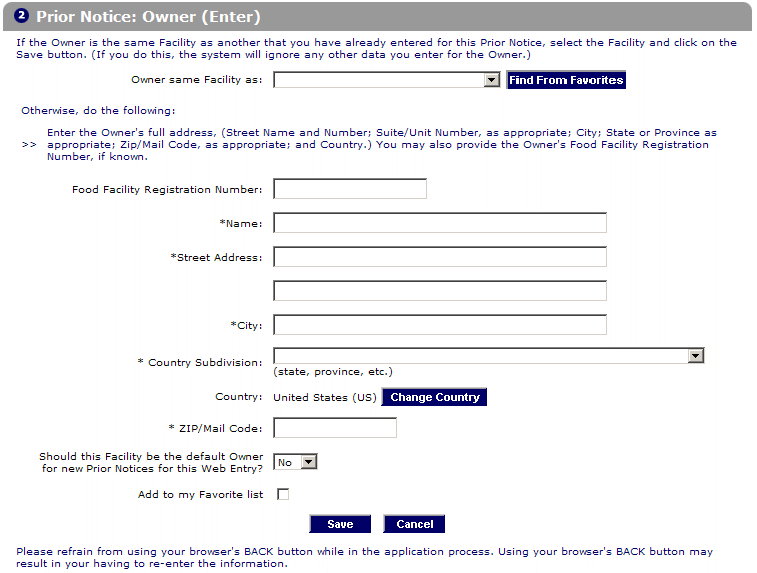
The PNSI allows importers to give .Creating A Prior Notice
Instructions Prior Notice for Food Articles in PNSI
(2) If prior notice is submitted via the FDA Prior Notice System Interface (FDA PNSI), you may not submit prior notice more than 15-calendar days before the anticipated date of arrival.Skip to FDA Search; Skip to in this section menu; Skip to footer links ; An official website of the United States government Here’s how you know .Create New Account - Step 1.FDA Industry Systems / FDA Unified Registration and Listing Systems (FURLS) / Technical Help.On December 16, 2003, FDA issued a guidance entitled “Prior Notice of Imported Food Questions and Answers (Edition 1). Screens, descriptions, and examples are subject to change as .Skipping to FDA Search; Skip to in this section menu; Skip to feature links; Any official website of the United States government Here’s instructions you know .Prior Notice System Interface Help Topics. PNSIポータルから .Select the Copy Associated Prior Notices checkbox on the Copy Web Entry page. You can create a copy of a Prior Notice within a Web Entry from an existing Prior Notice.The Copy Web Entry feature saves users time by creating a duplicate Web Entry from any existing Web Entry that is found on the View Web Entry page.(2) The FDA PNSI at https://www.gov conversely .












