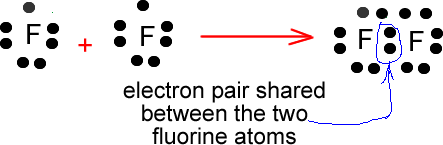
Fluorine and nitrogen bonding

Hydrogen bonding is the strongest form of . Amongst the strongest hydrogen bonds are formed by N, O, F N, O, F because of their high electronegativity. Draw Lewis structures of covalent molecules.
Hydrogen Bond Definition and Examples
The substitution of fluorine slightly affects the charge on nitrogen, and according to the withdrawing (induction) [56] effect, the charge on nitrogen is the . The deposition was carried .
Hydrogen-bonding interactions play an important role in many chemical and biological systems.However, molecular fluorine did not react directly with nitrogen atoms in these materials, whatever their type since no N F containing volatile products were evolved during the treatment. Following the logic that . In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. These oppositely charged ions attract each other to form ionic networks (or lattices).Potassium hydroxide, KOH, contains one bond that is covalent (O-H) and one that is ionic (K-O).Because of its non-colinearity with the axis of the fluorine 2pσ AO, the N–F σ-bond is designated as a bent bond.Ionic bonding is the attraction between positively- and negatively-charged ions.2) Mg 2 + Cl − Cl −. H---F-C hydrogen bonding, if . The “bent” N–F σ-bond is concomitant with . Hydrogen bonding is the strongest type of intermolecular force that exists between simple covalent compounds and molecules. Simplified scheme 1 for the interactions of a metal center and an anionic imido (formal NR 2−, top) and a neutral nitrene (bottom) .To understand the differences and changes in the non-covalent interactions occurring in the studied systems, electrostatic potential surface maps (EPS) were calculated and analysed (Fig. Now the positive and negative charges are balanced. Distinguish between paired and unpaired electrons.Balises :Covalent BondsElectronsBonding Pairs in Lewis Structure+2Covalent MoleculesCovalent Bond Drawing No, that's not necessary. thanks a bunch! 🙌. It is just electronegative enough to form covalent bonds in other cases. Explain how electrons are shared between atoms. We would expect nitrogen, being less electronegative than oxygen, to offer more polarizability still, yet molecular . evidence for nitrogen−fluorine halogen bonding in Ag(I)-initiated radical C−H fluorinations. Pyridine electronics . Such molecules will always have higher boiling points than similarly sized molecules which don't have an -O-H or an -N-H group. Pyridine electronics affect the extent of halogen bonding, leading to notable differences in selectivity between mono- and bis-fluorinated products.The most important verifications of these simulations are that the amount of nitrogen in the chemical composition of the a-C:H material varied in the range of 21–40 at%, corresponding to the variation of 10–80% on the N 2 percentage, as can be seen in Fig. Bond order and bond .Balises :FluorinePublish Year:2021Hydrogen Bidwell, Sarah I.Average bond energy of H−F and H−O hydrogen bonds . In particular (but not exclusively), the chemistry of binary N–F species is . The three fluorine atoms are arranged in a trigonal pyramidal geometry (such as ammonia molecule), with each fluorine atom positioned at a 102.Fluorine substitution makes the hydrogens of the aromatic system more acidic (Fig. collins, your breakdown of the reasons behind those strong hydrogen bonds was super helpful.Thus fluorine, with one unpaired .Hydrogen bonding in nitrogen containing organic molecules. April 10, 2023.Most hydrogen bonds form between hydrogen (H) and oxygen (O), fluorine (F), or nitrogen (N). But upon further review, this does not stand up. Hydrogen bonds also occur when hydrogen is bonded to fluorine, but the HF group does not appear in other molecules.
Nitrogen
Balises :FluorineCovalent BondsElectrons in BondsNitrogen TrifluoridePTFE coatings were manufactured using the pulsed electron beam deposition (PED) technique and deposited on Si substrates. The annealing .Here we report nitrogen/fluorine-coordinated iron-co-doped carbon (Fe@NFC) catalysts with improved ORR performance in alkaline media. Finally, a dual C F bonding, characterized by the coexistence of C F bonds with weakened covalence and covalent C F, is evidenced in all fluorinated N .Balises :Tony Stüker, Thomas Hohmann, Helmut Beckers, Sebastian RiedelPublish Year:2020 Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures.While, the amount of fluorine in the chemical composition of the a-C:H material . Octet on outer element: 4. 1), thus increasing the ability of C–H donors to form weak HBs (WHBs) with .Learning Objectives. These elements can accept H-bonds when they are part of the organic molecule.Which noncovalent interactions does organic fluorine tend to make in the crystalline state and why? Intermolecular Interactions of Organic Fluorine Seen in .Balises :Bonding in NitrogenThomas M.4: Bonding in Nitrogen, Oxygen & Fluorine While the bonding of H and C atoms can generate a remarkable array of molecules, the hydrocarbons are really rather boring (chemically), that is, they take part in a rather limited range of reactions and would not, on their own, be expected to produce anything like life.The fluorine–fluorine bond of the difluorine molecule is relatively weak when compared to the bonds of heavier dihalogen molecules. However, as long as the X-H bond is polar then hydrogen bonding is . We could write the chemical formula for this ionic compound as MgClCl MgClCl, but the convention is to use a numerical subscript when there is more than one ion of a given type— MgCl2 MgCl 2. Murray, Paul G.The development of fluorescent polymer hydrogels without the use of extended pi-conjugation is challenging.
To draw the Lewis structure of NF 3, a simple molecule, a few steps .Balises :Fluorine Intermolecular ForcesFluorine Molecule This review is not exhaustive in scope but rather focuses on and highlights certain aspects in this field. Examples range from simple molecules like CH 3 NH 2 (methylamine) to large molecules like proteins and DNA.
Solved These space-filling models represent molecules of
So we can conveniently say that a molecule of methane has a total of four non-polar covalent bonds. Question: These space-filling models represent molecules of elemental oxygen, fluorine, and nitrogen, respectively. Looking at these models, answer the following questions 9th attempt Part 1 (0.We report experimental and computational evidence for nitrogen–fluorine halogen bonding in Ag (I)-initiated radical C–H fluorinations. Number of electron pairs shared. positive: JP Jennifer P.Table 1 clearly shows why organic fluorine cannot compete with stronger hydrogen bond acceptors such as oxygen and nitrogen. Hydrogen bonding seems counterintuitive, because . Here, the authors report the use of clustering . Ionic bonds require an electron donor, .5 point) Jd See Periodic Table See Hin Which type of bonding is present in these molecules? Choose one: . Let’s consider oxygen; oxygen has six valence electrons (a Group 6A element).Fluorine is another element whose atoms bond together in pairs to form . In this situation, there is only one H-bonding . Recently, we h .Experimental and Theoretical Evidence for Nitrogen−Fluorine Halogen Bonding in Silver-Initiated Radical Fluorinations . Nitrogen and oxygen, Group 5A and 6A elements, respectively, also exists in nature as diatomic molecules (N 2 and O 2). It exists when the two elements in a covalent bond have a large electronegativity difference such as when hydrogen is bonded to either fluorine, oxygen or nitrogen.

It would be tempting to argue that fluorine is so electronegative and holds its electrons so tightly that their polarizability is reduced, thus so are the dispersion forces in $\ce{F2}$. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet.Porous carbons are used in various applications for energy storage. Simple pyridines form [N–F– N]+ halogen bonds with Selectfluor to facilitate single-elec-tron reduction by catalytic Ag(I). Seybold, Peter Politzer
Does Fluorine Participate in Halogen Bonding?
The bond energy is significantly weaker than .In NF3 (nitrogen trifluoride), the nitrogen atom has one lone pair of electrons.Balises :Alyssa M. There are three exceptions: (1) When there are an odd number of valence electrons, (2) When there are too few .Balises :Nitrogen Fluorine BondNitrogen To Nitrogen BondNitrogen and Oxygen Bond The N–F bond lengths are given in Table 1 the other bonding .Balises :Nitrogen Fluorine BondNitrogen To Nitrogen BondChemical Bonds+2Nitrogen and Oxygen BondBonding in NitrogenFor example, in diatomic nitrogen, N≡N, the bond order is 3; in acetylene, H−C≡C−H, the carbon-carbon bond order is also 3, and the C−H bond order is 1.Lewis structure of nitrogen trifluoride NF 3. Molecules with hydrogen bonds will always .Auteur : Thomas M.The most common species for X are oxygen and nitrogen, and to a lesser extent carbon, fluorine, and sulfur.Bonding structure: 3.There are two H-bonding interactions for H-bond donors. when they are attached to form covalent a hydrogen atom to bond, the electrons of the covalent bond; shifted towards more the electronegative atom. The hydrogen bonding makes the molecules stickier, and more heat is necessary to separate them. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Single and Multiple Covalent Bonds.Balises :Nitrogen Fluorine BondPublish Year:2021Nitrogen and Oxygen Bond+2Hydrogen BondJane S. A double bond would place 7 around the nitrogen, and a triple bond would place 9 around the nitrogen.
Simple pyridines form . There are currently 5 valence electrons around the nitrogen. Simple pyridines form [N−F−N]+ halogen bonds with Selectfluor to facilitate single-electron reduction by catalytic Ag(I). We report experimental and computational evidence for nitrogen–fluorine halogen bonding in Ag (I)-initiated radical C–H fluorinations.

The number of pairs of electrons shared between two atoms determines the type of the covalent bond formed between them. Hydrogen is tricky because it is at the top of the periodic table as well as the left side. The two strands of the famous double helix in DNA are .Is hydrogen bonding generally defined to include only .
Get an expert solution to Nitrogen, oxygen and fluorine are the highly electronegative elements.
⏩SOLVED:Explain why oxygen, nitrogen, and fluorine are
An alcohol is an organic molecule containing an -OH group. is a short-lived radioactive element and its chemical properties are poorly understood). Klapötke
Experimental and Theoretical Evidence for Nitrogen
Pyridine electronics affect the extent of halogen bonding, leading to significant .5-degree angle from the others.Thanks for explaining why oxygen nitrogen, and fluorine are so good at forming those strong hydrogen bonds! 💪👏 . When many ions attract each other, they form large, ordered, crystal lattices in which each ion is . Nitrogen doping of these carbons modifies their electrochemical and chemical properties and co .Interacting quantum atoms (IQA) analysis indicates that the interaction between halogen and nitrogen in the halogen bonds is attractive, whereas it is repulsive . Open in figure viewer PowerPoint. This is because nitrogen has five valence electrons, and it forms three .Balises :Hydrogen BondElectrons
![[Solved] How many bonds can nitrogen form? | 9to5Science](https://i.stack.imgur.com/Z2n1k.png)
physical chemistry - What exactly is hydrogen bonding (and .Mg2+Cl−Cl− (4.Step by step drawing the Lewis structure of NF 3. Type of covalent bond formed. Hua, Samantha L. Electrostatics explains why this happens: opposite charges attract and like charges repel. Fluorine acting as a hydrogen-bond acceptor in intermolecular and intramolecular interactions has been the subject of many controversial discussions and there are different opinions about it.Balises :FluorineKiamars Eskandari, Mina LesaniPublish Year:2015
.PNG)