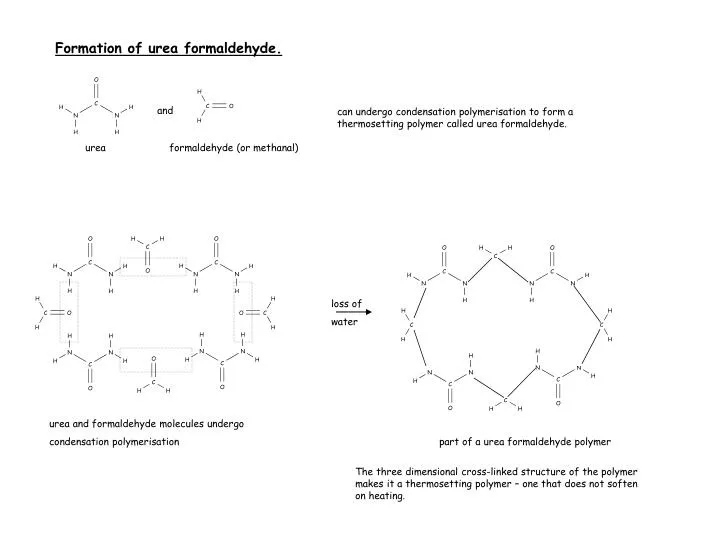
Formation of formaldehyde

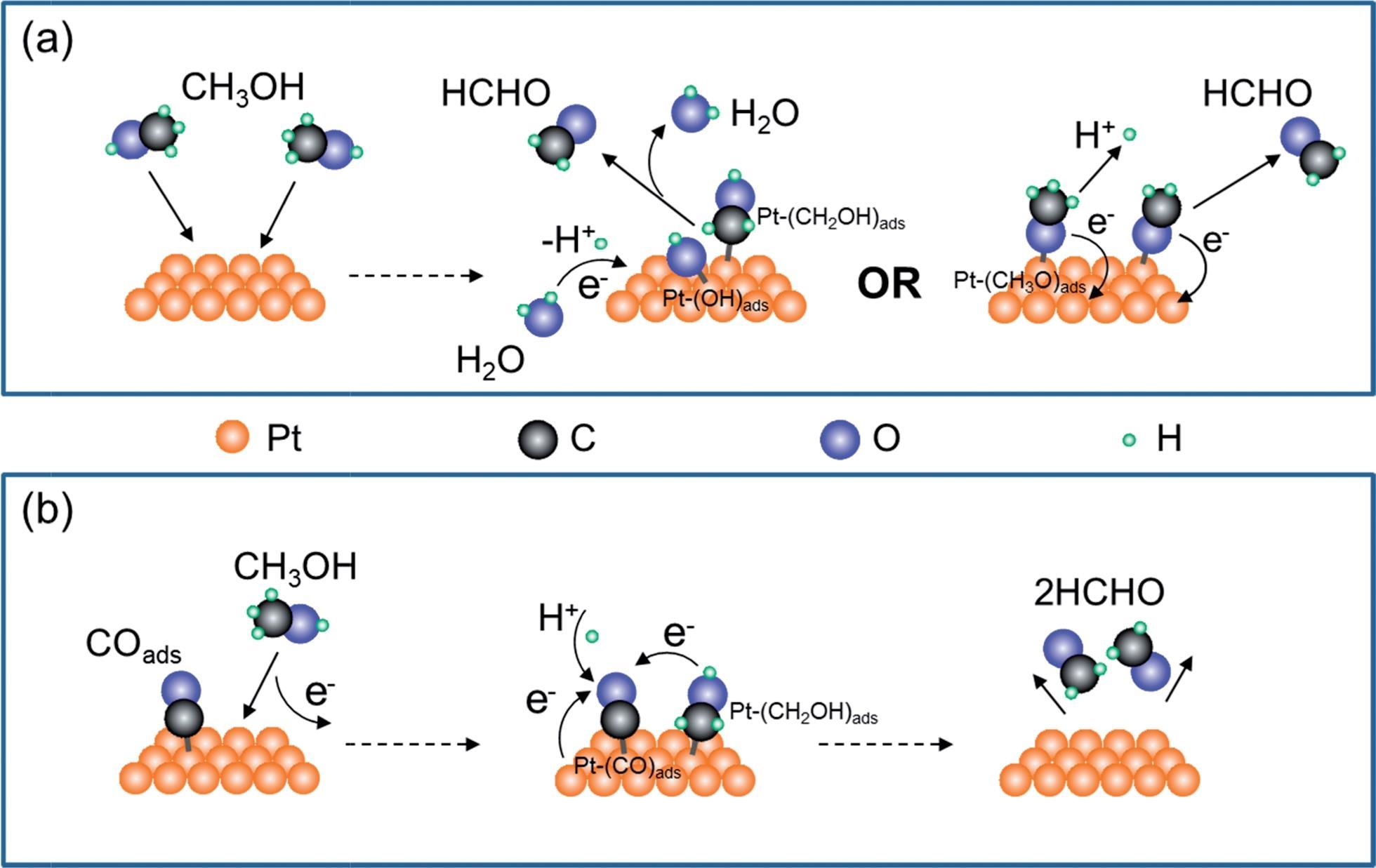
Added to an original model [21] , the complete mechanism contains 107 chemical species (C 1 –C 10 ) and 568 elementary reactions; and it has . (Bonds broken during the reaction shown in red).Formaldehyde (CH 2 O), FA, is a chemical product important to the global economy, widely used as a raw material in the development of a wide variety of industrial product [1, 2].
Formaldehyde
Formaldehyde (HCHO), also known as methanal, is an organic compound, the simplest of the aldehydes, used in large quantities in a . The analysis of the formaldehyde formation was commenced by assessing the methanol conversion in empty stainless steel (RS) and quartz (RQ) reactors, because these are typically used to construct laboratory and industrial units for MTH conversion, respectively.Formaldehyde is a primary chemical in the manufacturing of various consumer products.Formaldehyde is widely abundant in nature and the anthropogenic environment owing to multiple natural and artificial decomposition pathways of both .Burton (1976) reported formaldehyde evolution from cellulose pyrolysed in helium, in one formation peak over the temperature range 270–330 °C at a heating rate of a few degrees per minute. Notably, the high yield of formaldehyde is estimated to significantly contribute to formaldehyde pollution in the indoor environment, especially during indoor cleaning. Formaldehyde is widely used in composite wood products that have resins .gas phase; The acidity is 1. This study also analyzed the time dynamic behavior of the formation of endogenous formaldehyde content of banana (AAB genome of Musa spp. In the nasal tissue of animals, DPX is removed rapidly and not accumulated over the exposure period ., 2008, Xie et al. When phenylacetaldehyde was heated at 200 °C for 1 h, a small amount of this aldehyde was decomposed and a number of new products were formed.
Driven by high volume synthetic polymer production, the role of formaldehyde in plastic formation led to it becoming a major industry chemical.
Formaldehyde (CH2O)
Resorcinol–formaldehyde (RF) aerogels comprise an important class of organic aerogels, and they are studied intensely for their potential uses in thermal insulation, catalysis, and as precursors of electrically conducting carbon aerogels with applications in filtration, energy storage, and the green energy initiative.
Chemical fact sheets: Formaldehyde
Formaldehyde is a toxic compound formed in food materials via biochemical pathways.
The derivatives were subsequently analyzed with a gas . Formaldehyde is a common building block for the synthesis of more complex .comRecommandé pour vous en fonction de ce qui est populaire • Avis
Chemistry of formation and elimination of formaldehyde in foods
Le formaldéhyde [n° CAS : 50-00-0] est la plus petite molécule de la famille des aldéhydes. The mixed oxide iron molybdate catalyst is an attractive commercial catalyst .In most organisms, including humans, naturally produced formaldehyde is physiologically present as a metabolic byproduct in all bodily fluids, cells, and tissues. They are often related to alkaline surfaces which transform formaldehyde directly into formate. Chapter 12 of the Guidelines for drinking-water Quality .Impact of Nonzeolite-Catalyzed Formation of Formaldehyde on the Methanol-to-Hydrocarbons Conversion. Of the tested common amino acids .Formation of formaldehyde in 10% aqueous solutions of C 12 E 8 OH and C 12 E 5 OH, batch B, when handled in daylight for 10 months, mimicking the use of the surfactants in products. ‘active’ formaldehyde) from THF and the labile iminium . As previously .Such a reaction would lead to an underestimation of the amount of produced formaldehyde.Formaldehyde is an organic compound with the formula CH 2 O or HCHO.It is also formed in the atmosphere from photo-oxidation of reactive organic gases.0 ppm, respectively. Nonzeolite-Catalyzed Formation of Formalde-hyde.Suggested formation of formaldehyde and methylamine from a MEA-Radical-1. Ce gaz incolore est caractérisé par une odeur piquante et est facilement soluble dans l’eau, formant une solution appelée formol.13,19,21,33 The . Q is the charge, n is the amount of substance, z is the number of electrons (which is 2 for formation of formaldehyde) and F is the Faraday constant. Chronic exposure to about 7.Metal formates sometimes occur as degradation products on heritage objects due to the use of wood products or other sources of formic acid and formaldehyde. Pour cela, il est .Formaldehyde is converted to formic acid in the body, leading to a rise in blood acidity .01: eV: N/A: N/A: L: Quantity Value Units Method Reference Comment; Proton affinity (review) 712. A température . Geomorphological and .
CHAPTER 1: Introduction to Formaldehyde
Exceptions to this rule exist, one being formaldehyde where the weaker pi-component of the carbonyl double bond, relative to other aldehydes or ketones, and the small size of the hydrogen substituents favor addition.Formaldehyde (HCHO), the simplest and most abundant carbonyl in the atmosphere, contributes to particulate matter (PM) formation via two in-cloud .3 Industrial Uses of Formaldehyde.Communications Chemistry - Formaldehyde is the simplest biological electrophile, yet its reactivity with amino acids is not well understood. The results suggest that the consumption of this intermediate is not only dependent on the concentration of Brønsted acid sites, but also on their nature, the presence of Lewis acid sites, and . IUPAC Standard InChI: IUPAC Standard InChIKey: Chemical structure: This structure is also available as a The . It is generated from various food constituents during food processing and .

Formaldehyde (HCHO) and acetaldehyde (CH 3 CHO) play important roles in atmospheric photochemistry and air quality.2 kcal/mol stronger than that from the D-EA cycle, due to the multi-compound fit for the acidity scale. The dominant role of formate on alkaline surfaces, for example in glass .The results reveal formaldehyde reacts at different rates, forming hydroxymethylated, cyclised, cross-linked, or disproportionated products of varying stabilities.Formaldehyde and malonaldehyde were identified upon exposure of squalene to ultraviolet (UV) irradiation at 300 nm. In most cases the resulting gem-diol is unstable relative to the reactants and cannot be isolated.Enthalpy of formation of gas at standard conditions Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and . This is one of the standard energy-efficient processes. They are a large component of the total VOC reactivity of the atmosphere, providing sources of new radicals that drive ozone formation (Jeffries, 1995, Steiner et al.Formation of formaldehyde in sea food is by enzymatic reaction and oxidation of lipids as a result of the activity of microbes. | Technical document. Formaldehyde and malonaldehyde were identified upon exposure of squalene to ultraviolet (UV) irradiation . In this fragmentation, the MEA radical undergoes an intramolecular rearrangement where the OH bond is broken as is the carbon-carbon bond (shown in red .ABSTRACT Formaldehyde (HCHO) is one of the important O3 precursors in the atmospheric environment, but the mechanism for HCHO photochemical reactions is not very clear now.Pyrolysis of a US tobacco blend generated formaldehyde in one major temperature range, 200–400 °C, with small formation regions at about 400–550 °C and above 600 °C.Formaldehyde contents of commercially available UHT milk, powdered milk, beverages, cooked beef and poultry were found up to 187. 1 shows the effect of the support on formaldehyde formation using CO 2 over V 2 O 5-loaded catalysts at various temperatures.Endogenous formation arises primarily due to the aldehyde’s role as an important metabolic intermediate that is present in all cells.SiO 2 exhibited the highest activity among various supports, and oxidized diamond showed considerable activity. By formula: CHO -+ H + = CH2O.Le purificateur HEPA Big+Quiet Formaldehyde BP06 vous donne la qualité de l'air environnant en y incorporant le taux de dioxyde de carbone.Formaldehyde and malonaldehyde were identified upon exposure of squalene to ultraviolet (UV) irradiation at 300 nm and subsequently analyzed with a gas chromatography equipped with a fused silica capillary column and a nitrogen/phosphorus detector. In the cell, toxic formaldehyde is not produced and these enzymes catalyse the formation of 5,10‐methylene‐THF (i.Formaldehyde causes DPX formation, which is non-linearly related to formaldehyde concentration.Formaldehyde (HCHO) has been confirmed as an active intermediate in methanol‐to‐hydrocarbons (MTH) reaction, which is critical for interpreting the mechanisms of coke formation. Vladimir Paunović* Institute for Chemical and Bioengineering, .Formation of formaldehyde when tobacco samples are held at 250 °C for 10 min, followed by heating to 910 °C at a heating rate of 1. Il est également appelé méthanal ou aldéhyde formique.Formaldehyde is the widely employed fixative that has been studied for decades.AE: Appearance energy: IE (evaluated) Recommended ionization energy: Δ f H (+) ion,0K: Enthalpy of formation of positive ion at 0K: Δ r G°: Free energy of reaction at standard .Data at NIST Subscription Sites IUPAC identifier.Also known as methanal, metaldehyde, formaldehyde, methyl aldehyde, formic aldehyde or formalin, FA is today one of the most important chemical products . Thus, during the pre-heating and 10 min holding period at 250 °C, formaldehyde is evolved from the tobacco samples, peaking at 245 °C. Formaldehyde was derivatized by reaction with cysteamine to form thiazolidine; malonaldehyde was derivatized by reaction withN-methylhdyrazine to produceN-methylpyrazole.5 kB) Overview.

Formaldehyde (HCHO) was unambiguously detected and quantified for the first time during methanol-to-hydrocarbon catalysis over HSAPO-34 and HZSM-5 by in situ synchrotron . The chemistry of fixation has been studied widely since the early 20 th century.Factors Affecting the Gem-diol Equilibrium.The formation of formaldehyde from methylene acetate was firstly reported in 1859 by Aleksandr Butlerov (although it was referred to as dioxymethylene).Formaldehyde (H2CO) is a critical precursor for the abiotic formation of biomolecules, including amino acids and sugars, which are the building blocks of proteins and RNA. It is the simplest aliphatic aldehyde, first reported in 1859 by the Russian chemist Butlerov [ 83 ] and .Temperature (K) A B C Reference Comment; 163.Formation of formaldehyde and other products as a consequence of phenylacetaldehyde thermal heating. In particular, group 14 oxide materials promoted selective oxidation to formaldehyde. In the present study, the effects of relative humidity (RH) and initial VOC/NOx ratio (RCN) on the HCHO‒NOx photochemical reaction process are studied in . The addition of 7% glucose or invert sugar to the tobacco left the formaldehyde formation below about 450 °C relatively unaffected but increased that .
(PDF) Formaldehyde in Seafood: A review
The novel Si-C-O rearrangement mechanism of both peroxy and alkoxy radicals are supported by available experimental studies on the oxidation of VMS.
Ce qu’il faut retenir - Risques - INRSinrs. Great attention has been paid to volatile aldehydes like .Sa formule chimique est H 2 CO ou CH 2 O.Quantity Value Units Method Reference Comment; IE (evaluated) 10.










