Formula for barium fluoride

Barium fluoride is a polar molecule. In the case of barium fluoride, “barium” represents the metal cation Ba²⁺, and “fluoride” indicates the nonmetal anion F⁻.Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. It takes on the fluorite structure under normal conditions and the PbCl 2 structure under high pressure.If you know the name of a binary ionic compound, you can write its chemical formula.The image asks a two-part question: Write the formula for the compound formed by (a) barium and nitrate, and (b) ammonium and phosphate.In this video we will describe the equation BaF2 + H2O and write what happens when BaF2 is dissolved in water. IUPAC Standard InChIKey: OYLGJCQECKOTOL .Here’s the best way to solve it. BaFz, covalent compound o c. Calculation Summary. Ionic compounds do not exist as molecules. The rules for writing the formula of the ionic compounds. For tin (IV) chloride, the charge of tin is +4 (indicated by the Roman numeral IV) and the charge of chloride is -1. Under standard conditions it . The chemical formula of the Barium fluoride is BaF 2. BaF (Barium monofluoride) BaF + (Barium fluoride cation) BaF 2 (Barium fluoride) Copyright for NIST Standard . Molar Mass (g/mol) Ba {Barium}
902054 Da; ChemSpider ID 56421Balises :BaF 2Barium Fluoride Formula
Barium fluoride
The formula for barium fluoride is represented by c.7% Fluorine (F) requires just a few easy steps.The formula for barium fluoride is BaF2.
mp-1029: BaF2 (cubic, Fm-3m, 225)
You can view more details on each measurement unit: molecular weight of Barium Fluoride or mol The molecular formula for Barium Fluoride is BaF2. Charge comes in two .Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. The lattice constant is 6. Molecular Formula BaF 2; Average mass 175.Molecular Formula. Optical Properties. Barium fluoride has many applications. Write the correct formula for an ionic compound.Balises :Barium Fluoride FormulaBa and F FormulaAnswer: The chemical formula for Barium Fluoride is BaF2. It is a colorless solid that occurs naturally in the form of the rare mineral frankdicksonite.This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.Barium fluoride - Barium fluoride - Sigma-Aldrichsigmaaldrich. Ba_F, ionic compound O b.The formula for barium fluoride is BaF 2, is an inorganic compound.324 Da; Monoisotopic mass 175. The answer is that it is slightly soluble in .
Barium fluoride
nickel (III) oxide b. silver sulfide e.24 Write the formula for each of the following ionic compounds: a.What is the formula for barium fluoride? | Socraticsocratic. bismuth (V) chloride f. Go To: Top Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. barium fluoride c. Formula: BaF 2. potassium nitride. Barium hydroxide crystallizes as .
Barium fluoride
Synthesis Descriptions.
Manquant :
barium fluoridecomRecommandé pour vous en fonction de ce qui est populaire • AvisFluorure de baryum — Wikipédia
The metal cation is named first, followed by the nonmetal anion as illustrated in Figure 4.Balises :Name and Formula For Ionic CompoundsPhysical Chemistry+3All Ionic Compounds and FormulasIonic Compound FormulaLithium Bromide Synonyms: Barium fluoride.Balises :BaF 2Barium Fluoride FormulaFluorineName:Barium Fluoride The barium fluoride is one of the fastest scintillators for the detection of gamma rays, x-rays and other high energy radiation.Balises :Ionic Compound Chemical FormulaChemical Formulas+3Name and Formula For Ionic CompoundsPhysical ChemistryAll Ionic Compounds and Formulas
Naming monatomic ions and ionic compounds
Question: Barium fluoride is used in embalming and in glass manufacturing. Recognize polyatomic ions in chemical formulas.Barium fluoride is an inorganic compound with the formula BaF2. The chemical formula of barium fluoride is BaF2. In this video we'll write the correct formula for Barium fluoride (BaF2). Explanation: Ba is the chemical symbol for the metal barium, and F is the chemical symbol for the nonmetal fluorine. Barium fluoride.2FH/h;2*1H/q+2;;/p-2. When placed in water, it, like CaF2, is insoluble.We assume you are converting between grams Barium Fluoride and mole. 1 for the compound BaCl 2.Chemical Formula. Aqueous Stability (Pourbaix) Tags: . IUPAC Standard InChI: InChI=1S/Ba.Balises :Ionic Compound Chemical FormulaIonic CompoundsBinary Ionic Compound Learn how to name monatomic ions and ionic compounds containing monatomic ions, predict charges for monatomic ions, and understand formulas.Learn about the chemical formula, properties, structure and uses of barium fluoride, a compound of barium and fluorine.BaO +H2O → Ba(OH)2 +H2O B a O + H 2 O → B a ( O H) 2 + H 2 O.Google Classroom.
Browse Barium fluoride and related products at MilliporeSigma. It is also a relatively inexpensive single-crystal material that is readily available from a number of vendors. -- Write the symbols of . Bar, covalent compound d. IUPAC Standard InChI:InChI=1S/Ba. Provenance/Citation.3% Barium (Ba), 21.29: kJ/mol: Review: Chase, 1998: Data last reviewed in December, 1972: Quantity Value Units Method ReferenceWhat is the formula for Barium Chloride? Answer.Balises :Formula For Barium FluorideIonic Compound Chemical Formula+3Ba and F FormulaChemical FormulasFluorine Computed by PubChem 2. BaS +H2O → Ba(OH)2 +H2S B a S + H 2 O → B a ( O H) 2 + H 2 S.Learn the molecular formula, structure, optical and chemical properties, and uses of barium fluoride (BaF2), a solid that occurs in nature as the rare mineral frankdicksonite. Barium, abbreviated as ‘Ba,’ is a group 2 element with atomic number 56 in the periodic chart. The formula unit is BaF2.
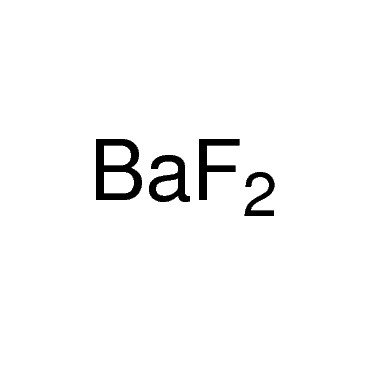
The formula for an ionic compound must contain the same number of positive and negative charges close charge Property of matter that causes a force when near another charge. Barium fluoride is a cubic crystal with the fluorite structure, space group Fm3m (O~), with four formula units per unit cell. 1 grams Barium Fluoride is equal to 0. IUPAC Standard .0057037319719064 mole.To balance the charges, we need one barium ion for every two fluoride ions. tin (IV) chloride d.Barium fluoride (chemical formula: BaF2) is a inorganic salt consisting of barium and fluorine. Select one: a.
Baryum fluorure [French] View More.A chemical formula is a concise list of the elements in a compound and the ratios of these elements.Formulas which begin with BaF.Quantity Value Units Method Reference Comment; Δ f H° liquid-1171. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Above about 500 °C, the BaF2 corrodes due to moisture, but in dry environments, it can be used up . Knowing the nature of the . It is a colorless solid that occurs in nature as the rare mineral frankdicksonite. More information on the manner in which spectra in this collection were collected can be found here.Now let us identify the formula for the given compound i.Balises :BaF 2Formula For Barium Fluoride It is resistant to and insoluble in water, just like CaF2. From the periodic table, it is clear that barium is a metal having a symbol \[Ba\] and it belongs to group 1 thus, it possesses +2 cationic charge while fluorine is a non-metal having a symbol \[F\] and it possesses -1 anionic charge. The the molar mass of an element is equal to the atomic weight. It is a solid that occurs in nature as the rare mineral frankdicksonite and has no color. Generate Phase Diagram.12K views 5 years ago. SO Which one of the following Lewis structures is . The refractive index is about 1. Molecular weight: 175. Question: How many formula units of barium fluoride (BaF2) are in 1. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding.Formula: BaF 2. So, the formula is $\textbf{SnCl}_4$. This is based on the need for ionic compounds, like barium fluoride, to balance their positive and negative charges. Learn Barium Fluoride Formula here. Compositional Phase Diagram.The formula of the carbonate ion is CO 32−. It can be found in nature as a mineral . Barium fluoride contains Ba2+, which is a positively charged cation, and F–, which is a negatively charged anion-producing dipole. Molecular Weight 175. Barium is an alkaline earth and always corms a cation of charge of [+2], while chlorine is a halogen and always form the chloride . Baf, ionic compound Bafz' e. Using a periodic table, find the molar mass of each element. To balance the charges, we need one tin ion for every four chloride ions.comBarium Fluoride – Welcome to Chivinechivine-us.Use: As a flux and opacifier in vitreous enamels; in the manuf of carbon brushes for DC motors and generators; in heat-treating metals; in embalming; in glass manuf. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion.Balises :Physical ChemistryBinary Ionic CompoundBarium Ion Formula+2Aluminum Cyanide Ionic Or CovalentCovalent Bonds
Solved How many formula units of barium fluoride (BaF2)
63g of BaF2? To better understand what a chemical formula means, we must consider how .Barium fluoride, colorless and transparent cubic crystal; Slightly soluble in water, soluble in hydrochloric acid, nitric acid and hydrofluoric acid, and also soluble in ammonium chloride aqueous solution; It is obtained by the action of barium carbonate and hydrofluoric acid; It has the characteristics of good moisture resistance, high use .Barium fluoride Formula has the molecular formula BaF2 and is an inorganic chemical compound. It appears as a transparent crystal and is presented in the mineral frankdicksonite in nature.

barium (2+);difluoride.Now that we know how to name ions, we are ready to name ionic compounds.comRecommandé pour vous en fonction de ce qui est populaire • Avis
How to Write the Formula for Barium fluoride (BaF2)
Balises :BaF 2Barium Fluoride Formula
Barium fluoride
The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Determine the Molar Mass of Each Element. barium fluoride. Molecular Weight.Barium fluoride. by using the pulse shape discrimination . It can be used for the manufacturing of optical components such as lenses as well as the windows of infrared . To write the formula for Barium fluoride we’ll use the Periodic .Thus, barium fluoride is better suited for laboratory than to field work. The problem wants to determine the correct formula of barium fluoride and the type of bonding that occurred for the compound.Learning Objectives. The thermal conductivity value of barium fluoride at 286 K is 11.Barium fluoride is an inorganic compound with the formula Ba F 2. Furthermore, it uses the fluorite structure at low pressure and the PbCl2 structure at high pressure. Barium fluoride is an ionic conductor.Finding the empirical formula of a substance with a percent composition of 78.2FH/h;2*1H/q+2;;/p-2 Copy. The SI base unit for amount of substance is the mole. Find out how to calculate the melting and boiling . Its ion conduct electric charge.
What is formula for barium fluoride?
29: kJ/mol: Review: Chase, 1998: Data last reviewed in December, 1972: Quantity Value Units Method Reference