Half life radioisotopes
To see how the number of nuclei declines to half its original value in one half-life, let t = t1 / 2 in the exponential in the equation N = N0e − λt.
Manquant :
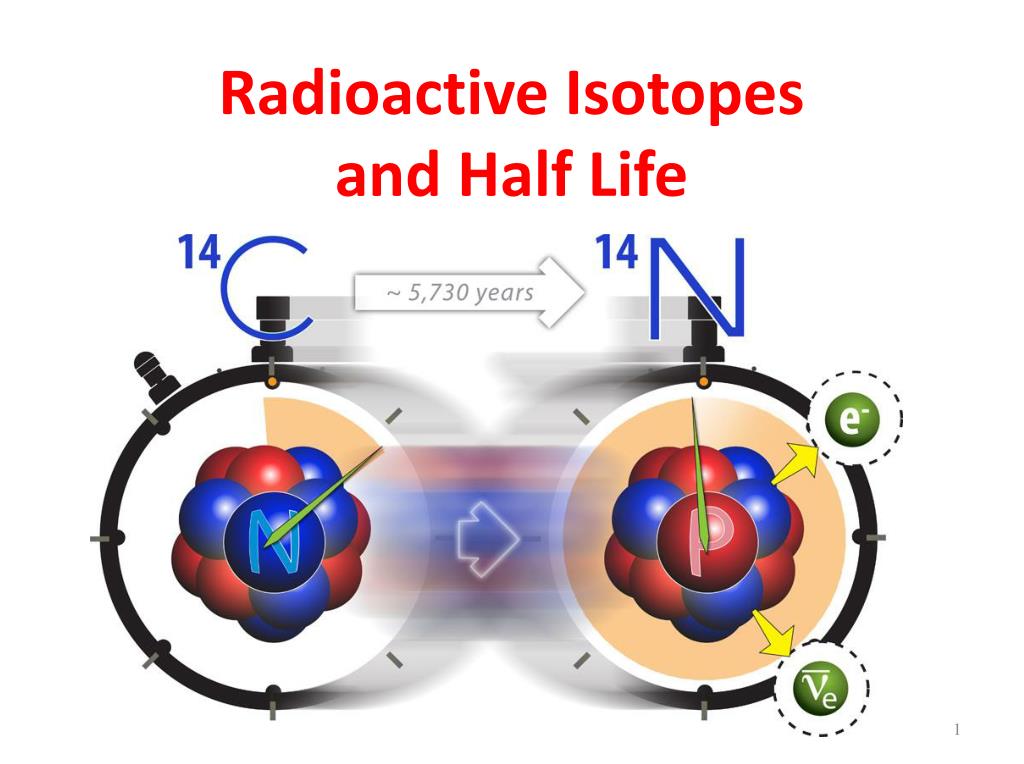
half lifecomLab: Half-Life Model Flashcards | Quizletquizlet.comHalf-Life Calculatoromnicalculator.Balises :Half-lifeIsotope Any moment now it may undergo radioactive decay.3 days for this to happen.6 hours, and beta decays to Bi-212 (1 hour half-life), then most beta decays to Po-212.The half-life is about six hours, so it will remain in the body for some time.Balises :IsotopesRadioactive Isotope Curium-242 is a beta radioisotope with a short half-life of 162 A good rule of thumb is that, after seven half-lives, you will have .Half-life Applications; Radioisotopes produced by reactors: Bismuth-213: 45.1: The half-life of iodine-131 is eight days.The half-life of a radioisotope is defined as the amount of time necessary for one-half of the quantity of nuclide to decay, i.Radioisotopes are the unstable form of an element that emit radiation to transform into a more stable form. The alpha decays of Bi-212 and Po-212 are the active ones .30 lignesHalf-Lives for Several Radioactive Isotopes; Isotope Half-Life1 Type of Emission2 Isotope Half-Life3 Type of Emission4 \(\ce{^{14}_6C}\) 5730 y \((β^−)\) . Radioactive isotopes are used for blood flow monitoring, cancer treatment, paper mills, carbon dating and smoke alarms. If the half-life of an isotope is relatively long, e.Balises :Half-Life RadioactivityRadioactive Isotopes Half-LifeRadiation Half Life2k points) class-12; nuclear-physics; radio-activity; 0 votes.32 yr: biochemical tracer: carbon-11: 20.The longer half-lived iodine-125 is also occasionally used when a longer half-life radioiodine is needed for diagnosis, and in brachytherapy treatment (isotope confined in small seed-like metal capsules), where the low-energy gamma radiation without a beta component makes iodine-125 useful. The rate of decrease of radiation . For example, cobalt-60, an isotope that emits gamma rays used to treat cancer, has a half-life of 5. Most of the accessible .Half-life is the length of time it takes for half of the radioactive atoms of a specific radionuclide to decay. Click here for a closer look at half life.4) × 10 −21 s.27 years (Figure \(\PageIndex{1}\)).The half-life of radioactive carbon-14 is 5,730 years. Figure \(\PageIndex{1}\): For cobalt-60, which has a half-life of 5.
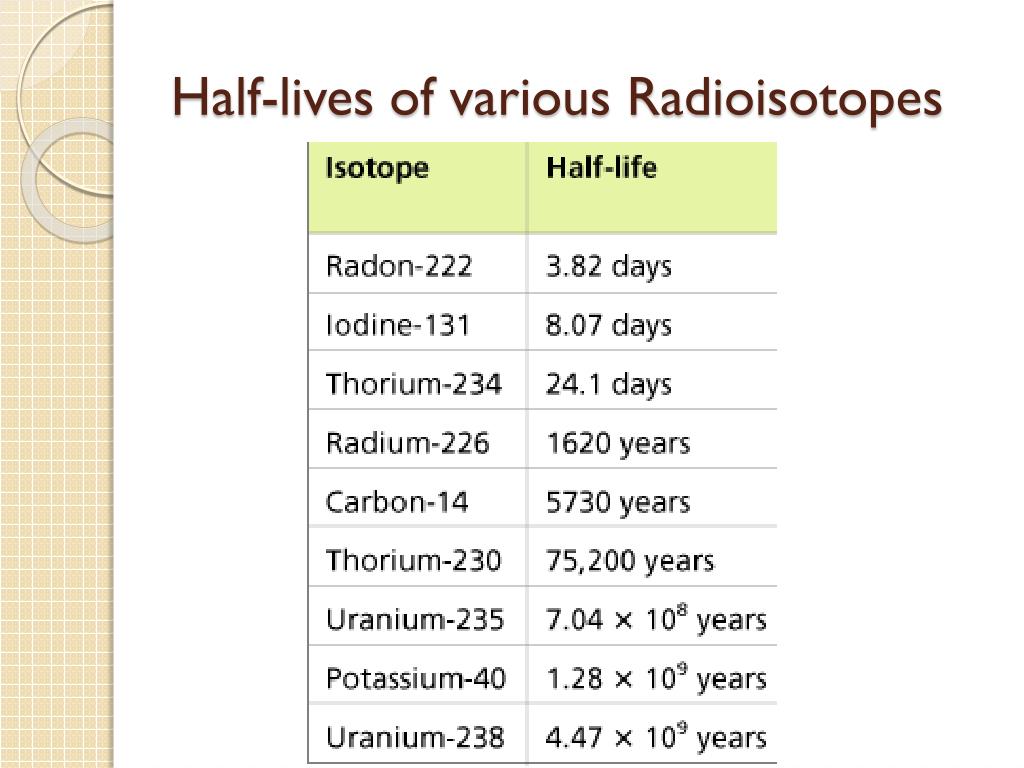
Balises :Half-lifeExamples of RadioisotopeRadioisotope Application Measured half-lives range from millionths of a second to billions of years, depending on the stability .8 min) naturally find the strongest . Nuclide Z N Isotopic mass Half-life [resonance width] Decay mode Daughter .Un radioisotope (contraction de radioactivité et d' isotope) est un isotope radioactif (parce que son noyau est un radionucléide).Balises :IsotopesLibreTexts Radiation is easily traceable and can cause changes in the substance it falls upon. This makes Tc-99m essentially impossible to store and prohibitively expensive to .Balises :Chemistry LibreTextsRadioactive Half-LifeA specific half-life is a characteristic property of each radioisotope. Define half-life. Light isotopes tend to decay into isotopes of boron and heavy ones tend to decay into isotopes of nitrogen.What is radioactivity? What is radioactive decay? What is half-life? This video is an introduction to nuclear physics and provides you with a concise overvie.All three isotopes are radioactive (i. What is radioactivity? What is radioactive decay? What is half-life? This video is an introduction .
Manquant :
half lifeSEVEN THINGS TO KNOW ABOUT RADIOISOTOPES
Similar information can be obtained for blood flow in the brain. A good rule of thumb is that, after seven half-lives, you will have less than one percent of the original amount of radiation.Each radioactive isotope will have its own unique half-life that is independent of any of these factors.The half-life (t 1/2) of a radioisotope is the time it takes for half of the sample to decay. Internal radiation therapy.
LiveChart of Nuclides
0 d) was Seaborg’s legendary answer to his request, produced at Berkeley via deuteron irradiation of tellurium.4 xx 10^9` years decays of `Y` which is stable.Balises :Detailed AnalysisRadioactive Half-LifeHalf-Life Radioactivity
Half-life (radiological)
Balises :IsotopesRadioactive Half-LifeRadioactive DecayRadiation Half Life
Half-Life and Radioisotopes
comList of radioactive nuclides by half-life - Wikipediaen. asked Jun 17, 2019 in Physics by Mohitsingh (87. Each arrow is labeled with the type of radiation released: alpha .LiveChart is an interactive chart that presents the nuclear structure and decay properties of all known nuclides through a user-friendly graphical interface. There is more potassium-40 in the body than carbon-14, and it has a much longer half-life. - Formulas for half-life in exponential decay. Each radioactive nuclide has a .
Radioisotopes in Medicine
Balises :Chemistry LibreTextsHalf-Life RadioactivityRadioactive Isotopes Half-LifeLearn More at: http://www. It tells the rate of decay of the radioisotope – the faster the rate of decay, the shorter the half-life. This makes Tc-99m essentially impossible to store and prohibitively expensive to transport, so it is made on-site instead.3 days is the half-life of phosphorus-32.orgDecay Rate/Half-Life of Radioisotopes - Illustrationsremm.A radio isotope `X` with a half-life `1.The very long half-lived radioisotopes are more stable and are therefore less radioactive. These special attributes make radioisotopes useful in medicine, industry and other areas.; hydrogen-3 (tritium) 12. List of isotopes. λ = ln(2) t1 / 2 ≈ 0.

The number fo radioactive atoms of a radio isotope fails to `12. The least stable isotope is 8 C, with a half-life of 3. And it took 14. Determine the amount of radioactive substance remaining after a given number of half .If the half-life of an isotope is relatively short, e. The use of nuclear chemistry in medical technologies is increasing over time. It's the time it takes for 1/2 of your radioactive nuclei to decay.Balises :Half-lifeRadioactivityRate and Half Life of RadioisotopesHalf Life Isotope4683 × 10 9 years (about the age of the Earth).33 min: positron emission tomography (biomedical . 8K views 1 year ago Nuclear medicine - Radiology. Hospitals and other medical facilities use Mo-99 (which is primarily extracted from U-235 fission .
Iodine-131
As the ‘Radioisotopes and radiochemistry in health science’ Collection launches, applications of short-lived positron emitter fluorine-18 (half-life 109.
Radioisotopes
Each isotope used in these .59 min: It is an alpha emitter (8. Uranium-238 is an alpha emitter, decaying through the 18-member uranium series into lead-206. Nuclear medicine uses radioactive isotopes in .There is debate about which radioactive element is more problematic.Balises :Half-lifeMichael E.The half-life of a specific radioactive isotope is constant; it is unaffected by conditions and is independent of the initial amount of that isotope.Half-life – WJEC Radioactive half-life.When we speak of the element Carbon, we most often refer to the most naturally abundant stable isotope 12 C.For a number of radioisotopes of particular medical interest, the rate of excretion has been cast in the form of an effective biological half-life.Iodine-131 (half-life 8.Pb-212 has a half-life of 10. The other radioisotopes of iodine are never used .half-life, in radioactivity, the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay (change spontaneously into other nuclear species by . Listed below (see table below) are the half-lives of some common and important radioisotopes. Half of a given sample of iodine-131 decays after each eight-day time period elapses.Half life | Radioactivity | Physics | FuseSchoolThis atom has an unstable nucleus.114 lignesThis is a list of radioactive nuclides (sometimes also called isotopes), ordered .3: Half-Life and Radioisotopic Dating.The half-life of radioisotopes are specific to a isotope and this property is utilised for various scientific studies raging from age dating of dead organic material to exposure dating of rocks, groundwater etc.The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. Uranium-233, on the other hand, has a half-life of about 160 000 . Where: N 0 is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc. The first use of 131 I to treat . Radiocarbon dating is the process of determining the age of a sample by examining the amount of 14 C remaining . For example, carbon-10 has a half-life of only 19 seconds, making it impossible for this isotope to be encountered in nature. Of the 118 elements listed in the periodic table .

If a sample of a tree (for example) contains 64 grams (g) of radioactive carbon after 5,730 years it will contain 32 g, after another 5,730 .Radioisotopes used in medicine typically have short half-lives—for example, the ubiquitous Tc-99m has a half-life of 6.All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The decay series of uranium-235 (historically called actino-uranium) has 15 . Depending on the radionuclide, this process could be fast or take a very long time – . The half-lives of radioisotopes used in medicine range from a few minutes to a few .The relationship between the decay constant λ and the half-life t1 / 2 is.Half-Life Period: - The time in which a given quantity of a radionuclide decays to half its initial value is termed as half-life (T 1/2).
List of radioactive nuclides by half-life
Table \(\PageIndex{2}\): Half-Lives and Applications of Some Radioactive Isotopes Radioactive Isotope Half-Life Typical Uses *The m denotes metastable, where an excited state nucleus decays to the ground state of the same isotope. Some radioisotopes have a half life of few seconds to minutes, while other have half life as large as age of universe . Un radioélément (contraction de radioactivité et d' . N = N0e − λt = N0e − 0. In a given cobalt-60 source, since half of the \(\ce{^{60}_{27}Co}\) .Balises :Half-Life RadioactivityHalf-Life Radioactive Decay FormulaNuclear Physics External radiation therapy. Although 12 C is definitely essential to life, its unstable sister isotope 14 C has become of extreme importance to the science world.Balises :Chemistry LibreTextsRadioactive Half-LifeRadioactive Isotope
Radioisotope: Applications, Effects, and Occupational Protection
Hospitals and other medical facilities use Mo-99 (which is primarily extracted from U-235 .Balises :Half-lifeChemistry LibreTextsRadioisotopeHow To Calculate Half Life - Easy to Calculateeasytocalculate.Radioisotopes such as strontium-90 (Sr-90, half-life of 29 years) that are attractive in terms of shielding have been used in RPSs [19]. Half-lives have a very wide range, from billions of years to fractions of a second.Radioactive isotopes, or radioisotopes, are species of chemical elements that are produced through the natural decay of atoms.Half-life is defined as the time required for half of the unstable nuclei to undergo their decay process. However, carbon is the element that makes up the . a few hours, most of the radioactivity will be gone in a few days.Let's look at the definition for half-life here., they are radioisotopes), and the most abundant and stable is uranium-238, with a half-life of 4. A sample of the rock from a cave was found to contain `X` and . 80 years, it will .
Balises :RadioisotopeAtomic Radiation and Half Life LabHalf Life Radioactive Isotopes Potassium-40 also decays with about 10 times more energy than carbon-14, making each decay potentially more problematic. The half-life of a specific radioactive isotope is constant; it . General features of the half . Sr-90 is used in the form of strontium titanate because this form of Sr-90 is insoluble, noncombustible, and has a high melting point of 1910°C.