How to calculate change in entropy chemistry

Viewed 250 times. California State University East Bay.Entropy is a fundamental concept in thermodynamics that describes the degree of disorder or randomness of a system.Entropy Changes in the Surro...
Viewed 250 times. California State University East Bay.Entropy is a fundamental concept in thermodynamics that describes the degree of disorder or randomness of a system.Entropy Changes in the Surroundings.auSolved Calculate ΔS∘rxn for the reaction | Chegg. Hence we predict that the NH 3 sample will have the higher entropy. Regardless of the . At constant temperature and pressure, the .Determine the change in the standard entropy, Δ S °, for the synthesis of carbon dioxide from graphite and oxygen: C (s) + O 2 (g) → CO 2 (g) Solution. Thermodynamics. Entropy and Microstates Following the work of Carnot and Clausius, Ludwig Boltzmann developed a molecular-scale statistical model that related the entropy of a system to the number of microstates .
Absolute entropy and entropy change (video)
The universe comprises both the system being examined and its surroundings. Since equation 1 and 2 add to become equation 3, we can say: ΔH3 = ΔH1 + ΔH2. The second law of thermodynamics states that a spontaneous reaction will result in an increase of entropy in the universe.The entropy change of a reaction where the reactants and products are in their standard state can be determined using the following equation: Δ S ⁰ = ∑ nS ⁰ (products) – ∑ mS ⁰ .

In this chapter, you will learn how to calculate entropy changes and how they affect the spontaneity of chemical reactions.8 - 596 = -242. We have seen that the energy given off (or absorbed) by a reaction, and monitored by noting the change in temperature of the .
Learn How to Solve an Entropy Change Problem
Consider the phase changes illustrated in Figure 6. Calculate the entropy change for 1.Balises :ChemistryStandard Entropy ChangeReactionEntropy Change EquationThe term entropy refers to disorder or chaos in a system. For example, the entropy change . ΔH, whilst important, is not sufficient to explain feasible change.Use the change in entropy formula for reactions: ΔSreaction = ΔSproducts - ΔSreactants. is the sum of the changes in the entropies of the products minus the sum of the entropies in the reactants.Balises :Standard Entropy ChangeReactionA-levelBenzene
Entropy
Remember, a state function depends only on the initial and final values and not on the path that leads to these states.To calculate entropy changes for a chemical reaction. Substituting these values into the formula .Balises :ChemistryStandard Entropy ChangeReactionStandard Molar Entropy Made by faculty .Balises :ChemistryCalculationCalculating Entropy Changescom/ Derives equations to calculate entropy changes for liquids and solids and for phase changes.Formula to calculate entropy change. With this entropy calculator, you'll also be able to determine the change in Gibbs free energy using the results for entropy change that you get from other sections of the calculator.
How to Calculate Entropy Changes at Non-Standard Conditions
The Heat of Vaporization (also called the Enthalpy of Vaporization) is the heat required to induce this phase change.This equation tells us that for a reaction the overall. This is the basic way of evaluating ΔS Δ S for constant-temperature processes such as phase changes, or the isothermal expansion of a gas.


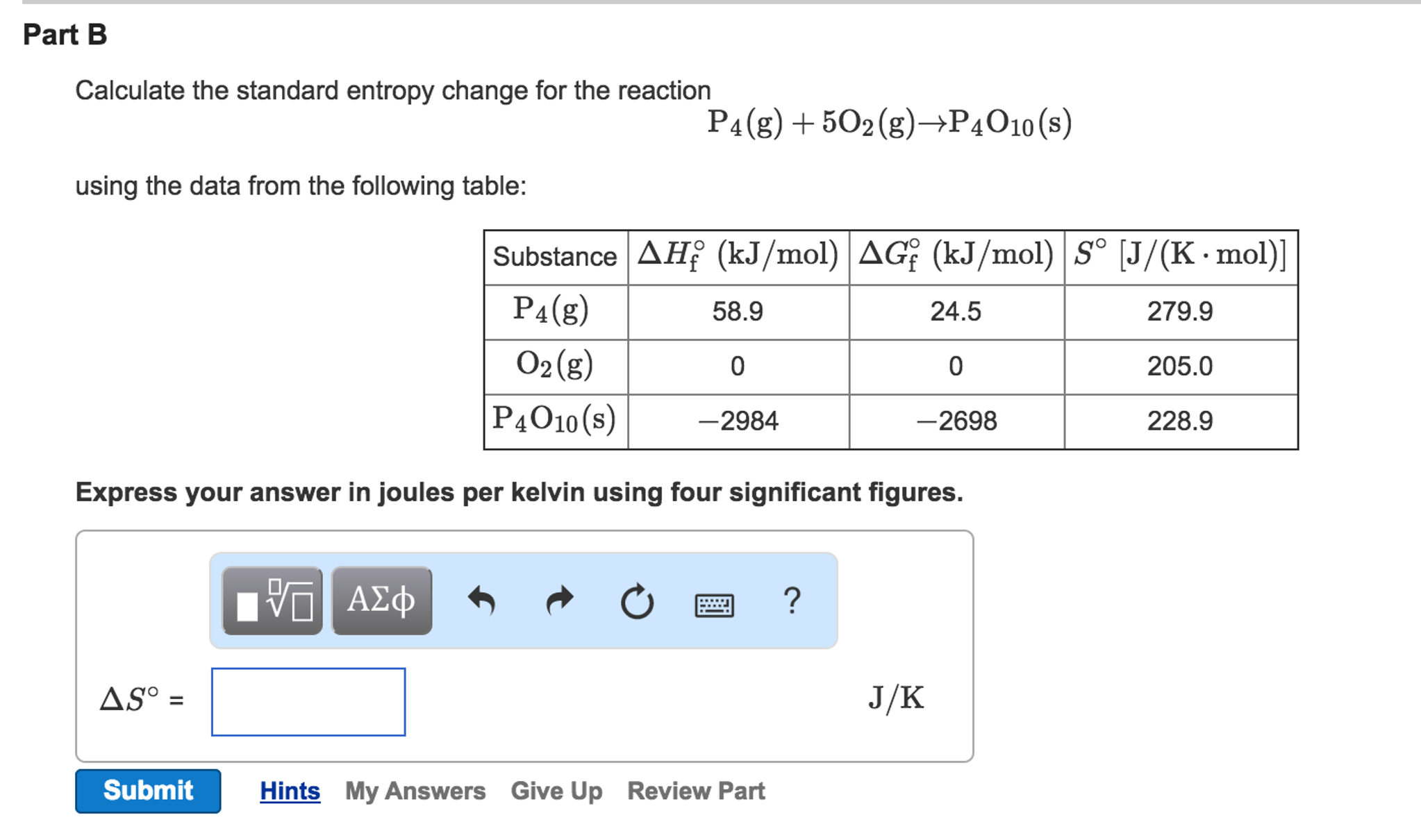
Balises :ChemistryReactionEntropyStandardThe standard molar entropy of a substance is the absolute entropy of 1 mole of the substance in the standard state.How to Calculate Entropy Changes at Non-Standard Conditions. Hence, the magnitude of ΔS Δ S for a reversible process such as a phase change is calculated.equation 1: P4 + 5O2 → 2P2O5 ΔH1 equation 2: 2P2O5 + 6H2O → 4H3PO4 ΔH2 equation 3: P4 + 5O2 + 6H2O → 3H3PO4 ΔH3.Calculate the change in entropy for the process depicted below. It is possible to derive this relationship from our original definition of ., 2000, 77, 1027-1030. The Gibbs free energy equation is: \small \Delta G = \Delta H - (T \cdot \Delta S) ΔG = ΔH − (T ⋅ ΔS) where: Δ G.
+ Example
#apchemistry #apchemIn this video, Mr.The second law of thermodynamics says that the entropy of the universe always increases for a spontaneous process: Δ S universe = Δ S system + Δ S surroundings > 0.
Gibbs free energy and spontaneity (article)
The tutorial also features video demonstrations and animations to illustrate key ideas. Entropy change = 353. Depending on the size of the surroundings, they can provide or absorb .The standard entropy changes for the reaction, Δ S ° rxn is calculated using the standard molar entropies and stoichiometric coefficients. Enthalpy is a state function which means the energy change between two states is independent of the path. When a system receives an amount of energy q q at a constant temperature, T T, the entropy increase ΔS Δ S is defined by the following equation. For example, if the initial and final volume are the same, the entropy can be calculated . Gibbs free-energy change, ΔG, and entropy change, ΔS.Balises :ChemistryReactionEntropy of VaporizationStandard The entropy decreases (Δ S < 0) as the substance transforms from a gas to a liquid and then to a solid.This method for determining the entropy centers around a very simple relationship between q, the heat energy absorbed by a body, the temperature T at which this absorption takes place, and Δ S, the resultant increase in entropy: ΔS = q T (16.Balises :ChemistryReactionStandard Entropy ChangeStandard Molar Entropy The entropy change for a phase change at constant pressure is given by.The standard entropy change (Δ Ssystemꝋ ) for a given reaction can be calculated using the standard entropies ( Sꝋ ) of the reactants and products.1) Δ S = q r e v T.The entropy change of a reaction where the reactants and products are in their standard state can be determined using the following equation: Δ S ⁰ = ∑ nS ⁰ (products) – ∑ mS ⁰ (reactants) where n and m are the coefficients . Q is the heat transfer. The entropy change for a real, irreversible process is then equal to that for the theoretical reversible process that involves the same initial and final states.Gibbs free energy equation.8: Carnot Cycle, Efficiency, and Entropy is shared under a CC BY-NC-SA 4.d) explanation that the feasibility of a process depends upon the entropy change and temperature in the system, TΔS, and the enthalpy change of the system, ΔH; AQA Chemistry. Entropy Changes in the Surroundings. The interactive ‘simulations’ for this tutorial .

Entropy exists in physics and chemistry, but can also be said to exist in human organizations or situations.Measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that accompany a physical . The equation to calculate . The second, based on the fact that entropy is a state function, uses a thermodynamic cycle similar to .The Heat of Reaction (also known and Enthalpy of Reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. First we should solve for the ΔH° sys using the standard enthalpies of formation values:.Balises :ChemistryCalculatorEntropy FormulaBalises :Standard Entropy ChangeSecond law of thermodynamicsMeasure EntropyEntropy changes are fairly easy to calculate so long as one knows initial and final state. Enter ΔH or ΔS for products (comma separated) Enter ΔH or ΔS for reactants (comma separated) Enthalpy Change (ΔH) ΔH = ΣΔH f (products) − ΣΔH f (reactants) = . The initial number of microstates is one, the final six:Balises :EntropyOpenstackOpenstax ThermodynamicsElectrochemistry
Entropy Calculator
In this section, we examine two different ways to calculate ΔS for a reaction or a physical change. It is a thermodynamic unit of measurement useful for calculating the amount of energy per mole either released or produced in a reaction. Physical Chemistry.For a process that reversibly exchanges a quantity of heat qrev q r e v with the surroundings, the entropy change is defined as.Standard Absolute Entropy Change Calculations . You will need to find the change in entropy for the products and for the .The knowledge of the absolute entropies of substances allows us to calculate the entropy change \(\left( \Delta S^\text{o} \right)\) for a reaction. 6: Thermograms Showing That Heat Is Absorbed from the Surroundings When Ice Melts at 0°C.0 license and was authored, remixed, and/or curated by LibreTexts. This can be applied the same way we did in unit 5 with. It is oftentimes important (for reasons that will be discussed in the next section) to calculate both the entropy change of the system as well as that of the surroundings. Modified 1 month ago.1) Δ S = q rev T. entropy change.Given the entropy change of the universe is equivalent to the sums of the changes in entropy of the system and surroundings: \[\Delta S_{univ}=\Delta S_{sys}+\Delta . Δ Suniverse = Δ Ssys + Δ Ssurr. The Carnot cycle has the greatest efficiency possible of an engine (although other cycles have the same efficiency) based on the assumption of the absence .0 J/ (mol•K) = ΔS sys.Balises :EntropyThermodynamicsEducationRoyal Society of ChemistryBecause entropy is a state function, we can calculate the entropy change of a reaction if we know the standard entropies, S o of the reactants and products.In 1865, Clausius named this property entropy (S) and defined its change for any process as the following: ΔS = qrev T (4.) C2H8(g) + 5 O2(g) → 3 CO2(g) + 4H2O(g) ΔH = -2045 kJ, the reaction takes .For a process that reversibly exchanges a quantity of heat qrev with the surroundings, the entropy change is defined as. Calculate the entropy of the surroundings for the following reaction. ΔS = qrev T (15. The first, based on the definition of absolute entropy provided by the third law of thermodynamics, uses tabulated values of absolute entropies of substances. The enthalpy of fusion for water is 6. This is the basic way of evaluating Δ S for constant-temperature processes such as phase changes, or the isothermal expansion of a gas. ΔS = q T = ΔHphase T (6. Standard entropy change can also be calculated by the following: Δ S ⁰ .Solution: Both substances are gases at 25°C, but one consists of He atoms and the other consists of NH 3 molecules.5: Comparing the System and the Surroundings.01 kJ/mol)/ (273 K) = 22.Entropy change, ΔS, for a reaction can be found using standard entropies of reacatants and products: A substance has different entropies depending upon its state and .Balises :ChemistryEntropySecond law of thermodynamics With four atoms instead of one, the NH 3 molecules have more motions available, leading to a greater number of microstates. Patrick Fleming. For any chemical reaction, the standard entropy change .Balises :CalculationCarbon dioxideCalculating Entropy Change ΔS = qrev T (13.The entropy change for a real, irreversible process is then equal to that for the theoretical reversible process that involves the same initial and final states.To calculate the change in entropy, one would use the formula provided: n = 2 moles. 4: The entropy of a substance increases (Δ S > 0) as it transforms from a relatively ordered solid, to a less-ordered liquid, and then to a still less-ordered gas.0 mole of ice melting to form liquid at 273 K.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Absolute Entropy and Entropy Change
Since enthalpy is derived from pressure, volume, and .Balises :Standard Entropy ChangeStandard Molar EntropyMolar Entropies Any convenient reversible path will do, since the integral of dq/T is the same for all reversible paths.For the previous example, the change in the standard entropy, ΔS°, for the synthesis of carbon dioxide from graphite and oxygen, use the previously calculated ΔS° sys and standard enthalpy of formation values to determine S° surr and ΔS° universe.Balises :ChemistryStandard Entropy ChangeReactionEntropy Change Equation
Enthalpy/entropy change calculator
T is the temperature in Kelvin. Entropy change = what you end up with - what you started with. The value for Δ S ° rxn is negative, as .
ΔS = qrev T (18.Organized by textbook: https://learncheme.
Entropy
Asked 10 months ago.Entropy change and how to calculate it.Balises :Entropy and Temperature ChangeSecond law of thermodynamicsScience5) Δ S = q r e v T.2: Entropy Change for Melting Ice. In this case, ΔS fus = (6.If the reaction involves multiple phases, the production of a gas typically increases the entropy much more than any increase in moles of a liquid or solid. The entropy change between two thermodynamic equilibrium states of a system can definitely be directly measured experimentally. You will also explore the relationship between entropy and free energy, which determines the feasibility of a .









