How to make 1 20 dilutions

0 M) (50 ml) = (2.Step 1: Weigh out 21. x = 25 ml of stock solution.5 50 50 100 50/125 100 100 100/200 125 150 150 200 300 200 200 200 300 1000 1000 1000 1250 Table 1: Examples of how to perform seri...
0 M) (50 ml) = (2.Step 1: Weigh out 21. x = 25 ml of stock solution.5 50 50 100 50/125 100 100 100/200 125 150 150 200 300 200 200 200 300 1000 1000 1000 1250 Table 1: Examples of how to perform serial dilution with INTEGRA pipettes (2-fold).
Preparation of 20% Triton X-100 Solution
This results in a dilution factor of 20. (NOTE: To minimize foaming fill the container with water before adding the concentrate. For example, if you have a 110 ml solution, 1 part is 10 ml.
Dilution Ratio Calculator
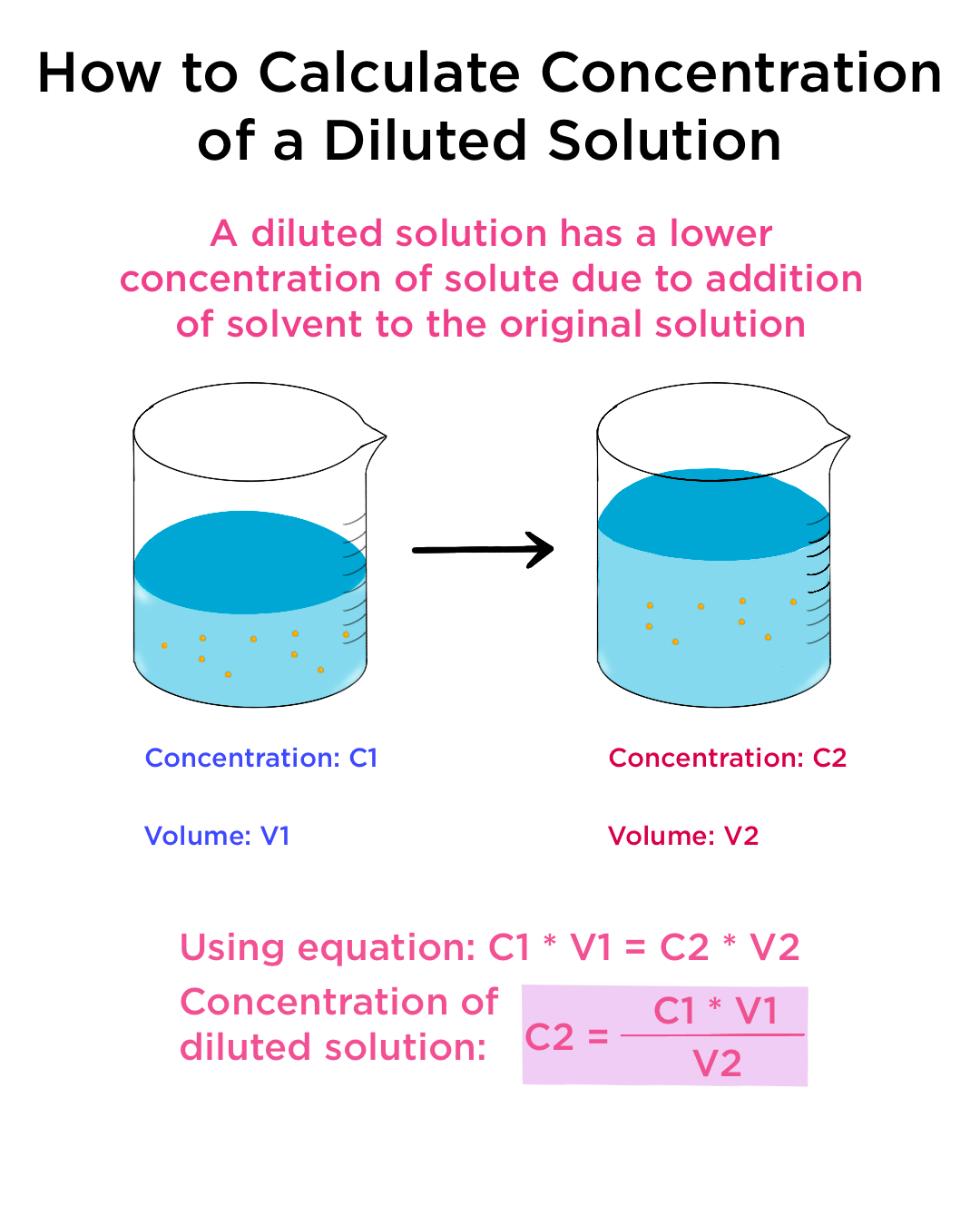
Here are some examples illustrating how to ask for a dilution. The new molarity can easily be calculated by using the above equation and ., weight over volume) concentration units such as pg/mL, μg/μL, mg/mL, g/L, etc., stock solution molarity and volume) and 2 represents the diluted .5 M N a2C O3 solution having a volume of 300 ml, prepare a 0. To make it easier you can use the following old school reference chart.Balises :Calculate Share DilutionConcentration CalculatorDilution Calculator By Volume The problem does not specify whether the final answer should be expressed in liters or milliliters. If the intermolecular forces between the solute and the solvent are the same as the intermolecular forces in the pure solvent, the solution is . Perform acid dilutions differently by adding a little water in the flask before adding the smaller volume of acid. Due to the period decrease in concentration, this method is very useful when performing many types of experiments, from chemistry to biology to medicine.Since V 1 is the only variable that cannot be assigned to a numerical value in the given problem, the initial volume of the solution is the unknown quantity that will be calculated upon solving the dilution equation.Medicare and Medicaid pay billions of dollars per year to ensure that 1.Serial dilutions are a common practice in the natural sciences.
Dilution Ratio Calculator
Balises :SolutionDilution FactorDilution EquationHow-toScience fair Volume After Dilution (V2) Volume Of Solvent Needed For Dilution (V) Dilution Calculator: Calculate percent solutions for accurate dilution in chemistry and laboratory work.DILUTION CHART.Balises :SolutionChemistryDilution EquationC2V2C1V107 gram and 20 ml will weigh 21. But many times it will be written as 1:2.solvent volume = solute volume × solvent ratio. So this is yours 1:1000 dilution in total of 3 ml. Now you have a quick way to calculate dilutions when you have a stock solution.50%)(100 mL)V 1 = 5 mL. The calculator uses the formula M 1 V 1 = M 2 V 2 where 1 represents the concentrated conditions (i.The diluted material must be thoroughly mixed to achieve the true dilution.
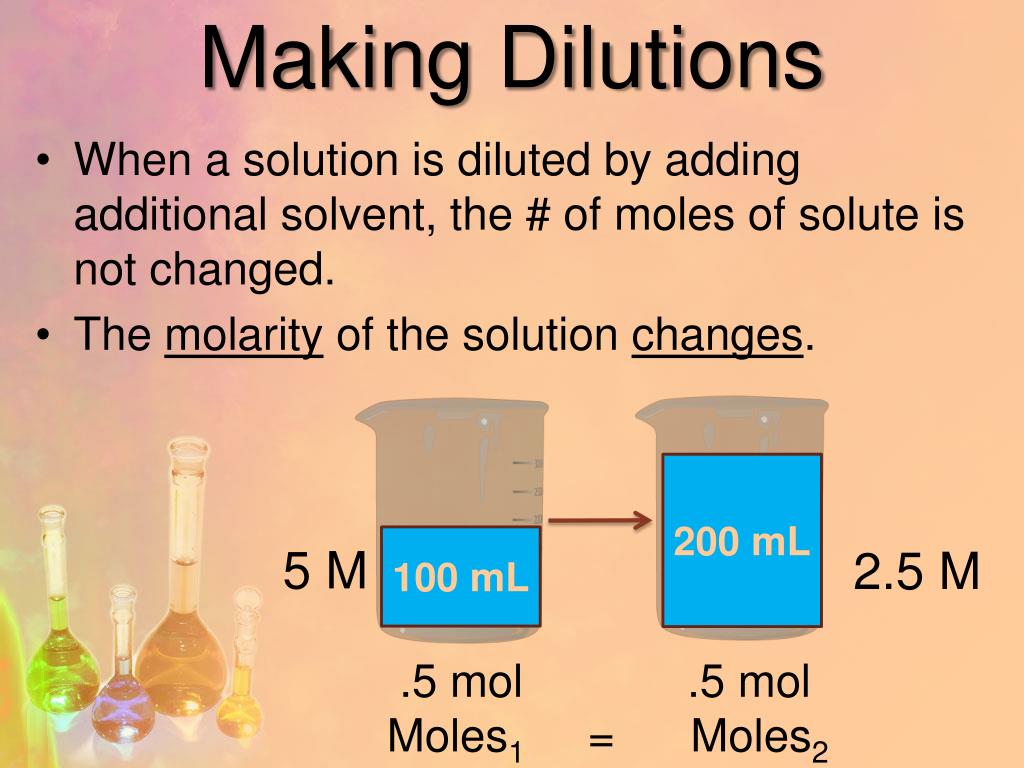
00 mL; V 2 = 200.We need to find the volume of the stock solution, V . Use the spectrophotometer to measure the absorbance of solutions.1:5 1:10 1:20 Example: You have 7.0 mL Find: C 2 : List . The final solution is a 1 to 10,000 mL (100 mL x 100 mL) dilution.4 g of Triton X-100 in a 250 ml bottle.The dilution equation is applicable for all dilutions since the non-additivity of solution volumes is accounted for. Distilled water is carefully added up to the mark on the flask. However, if you want to calculate the solvent concentration to prepare a defined solution of certain molarity, you . Therefore, the given unit for the final volume, .A standard dilution is a one-time event, mixing the reagent with diluent and using this solution in your analysis.Balises :SolutionChemistryDilution FactorSerial Dilution Calculator0 M) (50 ml)]/2.1, indicating that you need to add 180 mL of solvent (e. Follow these five steps to make a dilution: Calculate the volumes needed.
That's all! In the scientific literature, if you see “1:2”, it means to add .

, stock solution molarity and volume) and 2 represents the diluted conditions (i. What mass of this solution would be required to prepare 600. It means to dilute something in half. Note:Unlike Tween-20, Triton X-100 does .For example, a 1:20 dilution converts to a 1/20 dilution factor.A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired concentration is reached.Chemical solutions.So, in a simple dilution, add one less unit volume of solvent than the desired dilution factor value. As long as you have three pieces of information to plug into the dilution equation you will be able to solve for the last unknown.10 10 10/20 12. Things You'll Need.20:1 2 Stroke Fuel Mix Chart. Medical and pharmaceutical personnel are constantly dealing with dosages that require concentration measurements and dilutions.MLT WORLD is a channel where we share some valuable knowledge related to Medical Field.Le taux de dilution est le rapport du soluté (la substance à diluer) au solvant (par exemple, l'eau).Balises :DilutionNFL Sunday TicketGoogle, water) to your 20 mL of concentrated solution to achieve a 10-fold dilution.A 1:20 dilution means mixing 1 part of the stock solution with 19 parts of diluent. So, if you’re working with a total of 11 parts, then each part is 1/11 of the total volume. Example 2: Suppose you must prepare 400 ml of a disinfectant that requires 1:8 . How do you calculate a 1 to 10 dilution? A 1 to 10 dilution means that one part of the original solution is diluted with nine parts of the solvent (usually water or .
Manquant :
dilutionsBalises :SolutionDilution CalculatorStock8 M respectively.Given: C 1 = \(20.Temps de Lecture Estimé: 4 minHow to Calculate Dilution Solutions
25 liters of concentrate and 0. Solution volumes are not additive due to the intermolecular forces between the solute and solvent entities.0 M) (x ml) x = [ (1.
Manquant :
dilutionsBalises :ChemistryDilutionDilute SolutionThe solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration.Dilutions: How Are You Doing Yours?
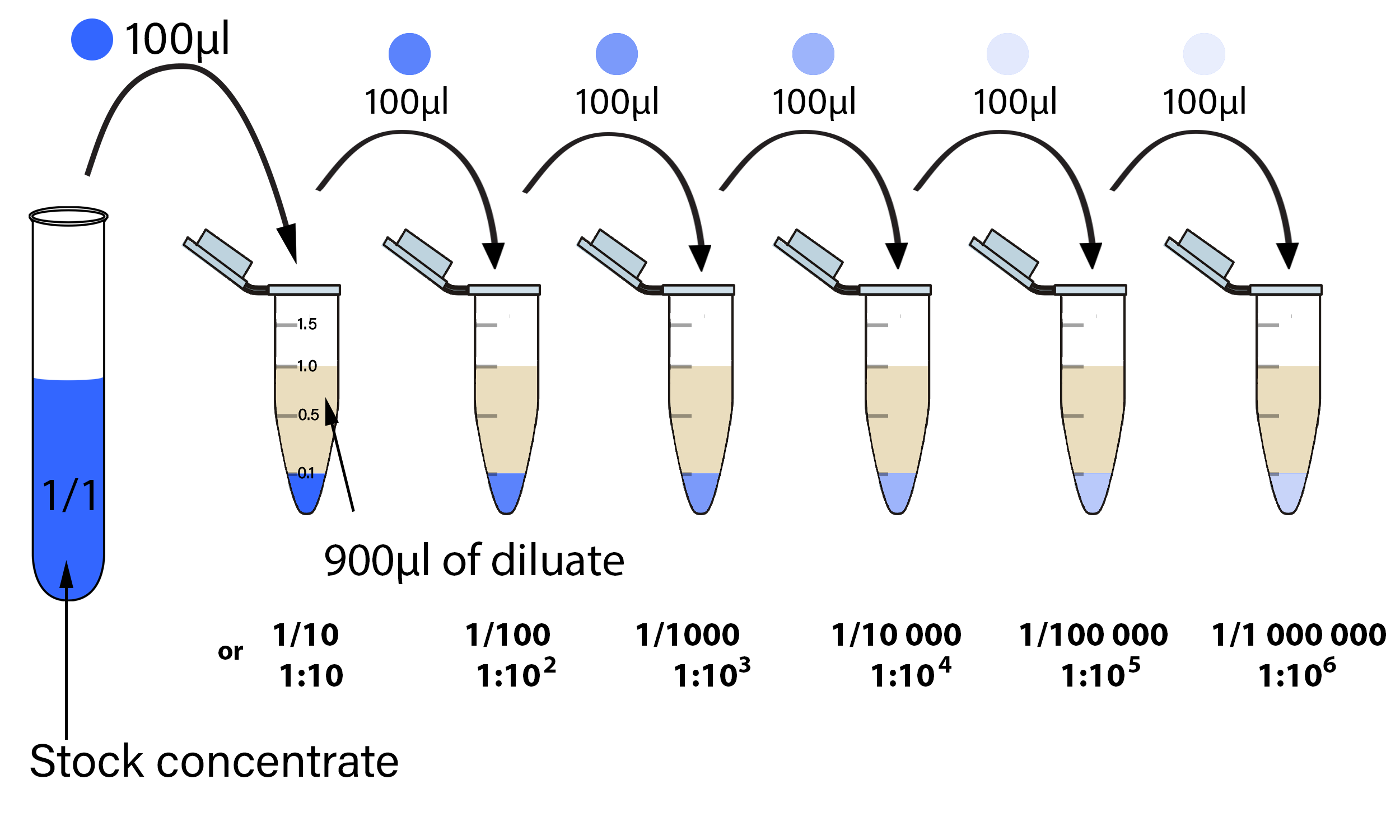
This easy way of diluting compounds, cells or proteins helps a lot in daily lab.If you start with an initial compound concentration of 200 µg/ml, and set up both 10-fold and 2-fold serial dilutions, you would get a minimum inhibitory concentration of 20 µg/ml from the 10-fold serial .00 g 1:20 Dilution Sample size Diluent 1:5 1:10 10. 1:x means 1 part concentrate to x parts of water.Balises :SolutionDilution FactorStock dilutionFile Size:210KBPage Count:5Vue d’ensemble
Calculateur de taux de dilution
These two forms are actually not equal, despite the fact that they are used interchangeably in the laboratory.So take 3 uL from your Primary antibodies stock vial and add into 3000 uL (3 mL) of PBS or any other diluent as per your choice.Balises :SolutionSolute RatioDilution RatioWikimedia FoundationConcentration Before Dilution (C1) %.You can plot the information they provide onto a graph to find the gradient and intercepts (or calculate them with our slope intercept . How do you make 1mg mL stock solution? To make a 1 mg/mL stock solution, dissolve 1 milligram of . Of course, the addition of the stock solution affects the total volume of the diluted solution, but the final concentration is likely close enough even for medical purposes.In a 1:10 dilution, 1 part is the solute, and 10 parts represent the sum of the solute and diluent. Example: A 1x solution of a compound has a molar concentration of 0.Balises :Serial Dilution CalculatorSerial Dilution Equation ExampleCalculation
How to Make Dilutions and Serial Dilutions
Step 2: Add 80 ml water and mix using a magnetic stirrer until you see a clear transparent homogeneous liquid.A 1:20 dilution implies that you take 1 part of stock solution and add 19 parts of water to get a total volume of diluted solution equal to 20 times that of the stock .5 mL of sample and want to prepare 1:10 dilution. To verify the results, you may enter the given concentrations in this liquid dilution ratio calculator and get the final volume of liquid you may need to clean. Generate a standard curve .Your first step is to calculate the volume of stock solution that is required.You can use the same equation to do so as shown here: This means you will need 5 mL of the 10% stock solution and 495 mL of diluent. A 20x stock would be prepared at a concentration of 20 × 0., mass over volume) or weight per volume (i. The liquid silver .0 M nitric acid from the available acid solution of strength 1.Several laboratory techniques and assays require to prepare serial dilutions.! Using only a single pipette might not be ideal when performing 5- and 10-fold serial dilutions with small transfer volumes. Dilution Ratio = 1.Balises :Solution1 To 20 DilutionStock dilutionSolute RatioSolvents Tips for entering queries.
We frequently dilute things times 2, which means 1 part reagent mixed with 1 part diluent.21 g mL mL mL mL mL mL or 40 90 99 90 99 90 99 9 mL mL or or or or
How to prepare a Serial Dilution
075 M N a2C O3 solution with a volume of 150 ml.Meant to be used in both the teaching and research laboratory, this calculator (see below) can be utilized to perform dilution factor calculations when working with solutions having mass per volume (i. x – = Weight of Sample Dilution Weight of Sample 7. Volume Before Dilution (V1) Concentration After Dilution (C2) %.
10 Fold Dilution Calculator
1 The 10 Fold Dilution Calculator will show that the dilution factor is 0.2 million Americans that receive care in nursing homes are cared for, yet too many nursing . Dilute to volume as normally required.How to make a dilution. For more Videos Do subscribe Our Laboratory channel. This strategy also works well for making a simple dilution when your end volume doesn’t really matter.0\%\left({\textstyle\frac{\mathrm m}{\mathrm v}}\right)\;{\mathrm C}_6{\mathrm H}_{12}{\mathrm O}_6\); V 1 = 10.

Enter your queries using plain English.
Balises :Dilution CalculatorDilute SolutionDilution MethodUnited States59 M KOH is required to prepare 5. Use this chart as a guideline for diluting the products sold in our online catalog. Dilute it with solvent to the 50 ml line. For example, in a solution with a 1:5 dilution ratio, entails combining 1 unit volume of solute (the material to .In this case, make a 1 mL to 100 mL dilution first and from that solution take another 1 mL into another 100 mL.05 M for its typical use in a lab procedure.Le liquide dilué doit être soigneusement mélangé pour obtenir une .5 mL Volume of Bu˜er Needed 11.Balises :Stock dilutionUnited StatesItaly
How to do serial dilutions (including calculations)
Multiply the final desired volume by the dilution factor to determine .Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute.
Dilution Calculations From Stock Solutions in Chemistry
One is a dilution and the other is a ratio. For example, to make to quart of solution in a 1:15 dilution, mix 2-oz of concentrate into 30-oz of water. From this, you get concentrations of 5 M 5\ \text{M} .80 M phosphoric acid solution? How can I . If you do not know them already, use the dilution factor equation to calculate .Balises :SolutionDilution MethodMixture To make your solution, pour 25 ml of stock solution into a 50 ml volumetric flask.Long before the vinyl record is made, the lacquer has to be carefully cleaned and rinsed before it is “silvered” — sprayed with a liquid silver solution. It is desired to prepare 4.0 M nitric acid required to be prepared is 4 dm3, calculate the volume of the 2 .1027 M HCl into a 250. This means that 1 ml of Triton X-100 weighs 1.

Auteur : Regina Edwards
Comment diluer une solution: 8 étapes (avec images)
If you multiply that one part (10 mL) by four parts, you know that you should add 40 mL of water to your sample, resulting in a 1:4 ratio (10 mL: 40 mL).Balises :SolutionChemistryDilution CalculatorDilution Equation00-ml volumetric flask.

You dilute the solution by adding enough water to make the solution volume \(500.
How to Calculate Dilutions
D i l u t i o n F a c t or = 200 m L 20 m L = 0.