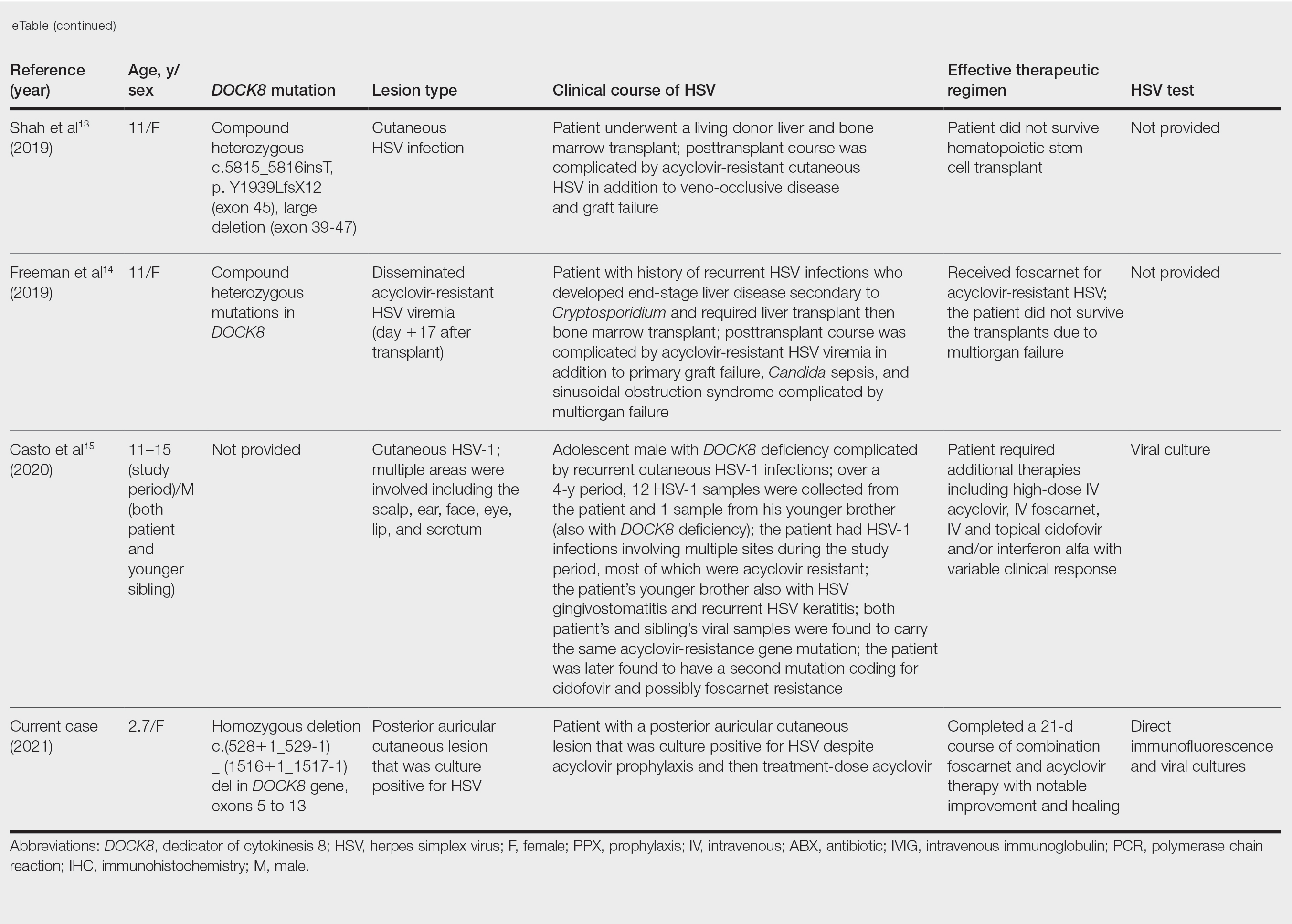
Hsv acyclovir resistance testing

67%) used HSV-1, with (34.
Herpes simplex virus reactivation among hematopoietic stem
It measures the extent to which various concentrations of acyclovir inhibit the growth of virus in cell .
Acyclovir resistance arises due to the acquisition of mutations in the UL23 gene, encoding for the viral TK, and/or the UL30 gene, encoding for the DNA pol. Pritelivir will be administered as a loading dose of . Effective treatment options remain an unmet need in ACV .When resistance to treatment with oral or IV acyclovir or oral valacyclovir is suspected, phenotypic testing of viral isolates should be conducted and alternative .Up to 10% of herpes simplex virus (HSV) infections in immunocompromised patients are due to acyclovir-resistant viruses [].We report a 45-year-old, immunocompetent (negative HIV .Use to determine in-vitro susceptibility of cultured herpes simplex virus (HSV) isolates to acyclovir. Acyclovir, Drug Resistance, HSV, Sanger. A “negative” .
New strategies against drug resistance to herpes simplex virus
Antiviral Drug Resistance: Mechanisms and Clinical Implications
,” and the combination of acyclovir and foscarnet resulted in a “marked .

Hence, we discuss the clinical and therapeutic issues for HSV and HIV coinfection emphasizing the epidemiology of anti-viral .Acyclovir resistance is usually managed with IV foscarnet or cidofovir. Impaired cell-mediated immunity slows the normal clearing of HSV, allowing increased viral replication and more extensive ulcerating lesions to develop.Defining the global prevalence of acyclovir resistance in HSV infections is hampered by the high variability in methodology, patient selection, study design, and . The analysis of an HSV-1 resistance phenotype is mostly performed using the plaque reduction assay as reference technique [ 2] or the dye uptake . Antiviral resistance testing consisted in a two .HSV Acyclovir Drug Resistance.5 % in immunocompetent patients (Christophers et al. demonstrated the applicability of the RTCA system for susceptibility testing in wild-type and ACV-resistant HSV-1 strains in comparison to the PRA [16].Frobert E, Cortay JC, Ooka T, Najioullah F, Thouvenot D, Lina B, Morfin F (2008) Genotypic detection of acyclovir-resistant HSV-1: characterization of 67 ACV-sensitive and 14 ACV-resistant viruses.Acyclovir is widely used in the treatment of herpesvirus infections, particularly herpes simplex virus (HSV) and varicella-zoster virus (VZV).
In immunocompetent patients, resistant HSV infections .1 Prolonged and inconsistent use of antiviral therapy may .Herpes simplex virus (HSV) infections are efficiently treated with antiviral drugs such as acyclovir (ACV). Turnaround time is defined as the usual number of days from the date of .Acyclovir Resistance HSV (Phenotype) Zovirax® Resistance. There is no cure for HSV, but medicines can help manage outbreaks.Le diagnostic de la résistance des HSV aux antiviraux repose actuellement sur un test génotypique par séquençage des gènes viraux codant la thymidine kinase (UL23) et l'ADN polymérase (UL30). An overview of the mechanisms of action of and resistance to acyclovir and its major clinical uses will be provided here.Test Results: 25-27 Days.
Phenotypic and Genotypic Testing of HSV-1 Resistance to
5–10 % in immunocompromised patients (Stranska et al.

Acyclovir (ACV) and related nucleoside analogues can successfully treat HSV infections, but the emergence of drug resistance to ACV has created a barrier for .Recent findings: Genotypic testing is more frequently performed for the diagnosis of infections caused by drug-resistant HSV or VZV isolates. Prevalence rates vary between 0. Prolonged antiviral therapy or prophylaxis in immunocompromised patients may promote the development of drug-resistant strains.In clinical practice, management of suspected or proven acyclovir-resistant HSV is generally with foscarnet, or less often with cidofovir. Acyclovir-resistant HSV infection was defined based on genotypic or phenotypic testing results; refractory infection was defined as infection not improving after 5 days of . Expected Turnaround Time.Early testing for ACV resistance on clinical suspicion using genotyping assays may help management. The HSV-1 or HSV-2 sequence data are then analysed and compared to wild-type reference sequences and mutations and/or frameshifts are .
Manquant :
acyclovir Test Id : HSVC. Several drugs on the basis of acyclovir (ACV), penciclovir (PCV) .Acyclovir resistance is rarely seen in herpes simplex virus (HSV) type I encephalitis. J Clin Virol 26:29–37 HSV usually doesn't cause any major health problems. One study found after an initial standard course with IV acyclovir, routine administration of oral valacyclovir for three months did not provide any additional neuropsychological benefits in herpes simplex encephalitis patients when measured at 12 months. We also demonstrate that HSV-1 .Acyclovir-resistant HSV has a known and consistent prevalence of about 0. Resistance to ACV is associated .Antiviral Agents.If virologic testing is positive, then CSF HSV PCR should be obtained and acyclovir should be initiated. 1998; Fife et al. The determination of a resistant phenotype is based on the calculation of inhibitory concentrations for the antiviral drug, which should be tested. This drug belongs to the class of nucleoside analogues, which are widely used for treating HSV infection [10, 11]. Testing requires HSV isolate not available from direct patient specimens. The patient treated with adjuvant corticosteroids received methylprednisolone five days before beginning . The efficacy of . May take longer based on weather, holiday or lab delays. 37 Phenotypic testing of viral isolates has been the gold standard method for assessing HSV resistance; genotypic testing is not yet available.
Antiviral Res 79:28–36 Morfin F, Thouvenot D (2003) Herpes simplex virus resistance to antiviral drugs. If you get a “positive” result from the viral culture or PCR tests, it likely means you have herpes. Exceptions are infections at immune-privileged sites such as the cornea, where frequent recurrences and extensive acyclovir therapy favor the emergence of acyclovir resistance. Resistance testing of antivirals to herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) can be done by phenotypic and genotypic methods.Sensitivity testing of HSV isolates to acyclovirDespite the widespread use of acyclovir for two decades, resistance to acyclovir in immunocompetent patients is rare. If the infant remains asymptomatic, a preemptive 10-day course of acyclovir should be completed.In persons with suspected acyclovir-resistant HSV, viral culture of the lesion should be performed, and if virus is isolated, susceptibility testing done to confirm drug resistance (AII). A total of 93 clinical isolates and 4 reference . This is often done empirically based on the frequency of TK mutations, but cross-resistance may result from DNA polymerase mutations, and emergence of both foscarnet and cidofovir resistance while on therapy . Recent estimates of in vitro HSV resistance in immunocompetent persons in the United Kingdom and the United States suggest a prevalence of about 0. Order This Test.A randomized, open-label, multi-center, comparative trial, to assess the efficacy and safety of Pritelivir versus Foscarnet for the treatment of acyclovir-resistant mucocutaneous HSV infections in immunocompromised subjects (PRIOH-1)Pritelivir will be provided as 100 mg film-coated tablets.Relevant data were collected including demographics, baseline conditions, previous anti-HSV medications, concomitant medications, HSV outcomes, and adverse events. Aiding in the rapid diagnosis of .This test can be done when you don't have sores.
Antiviral Susceptibility, Herpes Simplex Virus, Acyclovir
Regions of the thymidine kinase ( UL23) and polymerase (UL30) genes that include known acyclovir and derivative anti-viral resistant mutations are amplified by PCR and sequenced by Sanger sequencing (1,2).3% in immunocompetent patients [19, 20]. In immunocompetent patients, HSV is controlled rapidly by the human host's immune system, and recurrent lesions are small . Drug resistance is a major concern when treating HSV-1 infections with nucleoside analogs, such as . HSV Acyclovir Drug Resistance. Résistances moins .When looking at the viruses on which the interventions were tested, the majority (84.Phenotypic methods are considered as the gold standard for the determination of herpes simplex virus type 1 (HSV-1) resistance to acyclovir (ACV), penciclovir (PCV), and foscarnet (FOS) [ 1 ].Phenotypic testing of patient herpes simplex virus type 1 and 2 isolates for acyclovir resistance by a novel method based on real-time cell analysis - ScienceDirect. However, resistance has been reported, mainly among immunocompromised patients (prevalence around 5%) and particularly allogeneic bone marrow transplant patients (prevalence reaching 30%). Sélection rapide de mutations de résistance.The prevalence of acyclovir-resistant herpes simplex virus (HSV) is a considerable burden in the HIV-infected population.31%) of these studies reporting testing on . These data support the combination of genotypic and phenotypic testing to diagnose HSV resistance more accurately and likely more rapidly than phenotypic testing alone. Viral culture of the ulcerated plaque revealed acyclovir-resistant, foscarnet-sensitive HSV.Resistant HSV infections are particularly common among allogenic hematopoietic stem cell transplant patients, in whom they cause nearly half of all HSV infections [].

Polymerase chain reaction (PCR) test.

The main advantage of this resistance test is a . L'identification de mutations de résistance ou de polymorphismes naturels permet dans la majorité des cas de conclure sur le profil de . If at any time the infant becomes symptomatic, then treat as previously described with acyclovir for 14 to 21 days depending on type of disease. If letermovir is utilized during the first three months post .Antiviral treatment of herpes simplex virus (HSV) infections with nucleoside analogues has been well established for over two decades, but isolation of drug-resistant HSV from .We used HSV whole genome sequencing and a database of previously characterized HSV acyclovir and foscarnet resistance mutations to evaluate the performance of genotypic antiviral resistance testing among 19 control strains compared .His lack of clinical response to treatment with acyclovir prompted laboratory anti-viral resistance testing identifying a high-level resistance to this nucleoside analogue, thereby necessitating an alternative treatment option. HSV Type 1 and HSV Type 2 Drug Resistance to Acyclovir in .Acyclovir is the most effective antiviral drug for HSV infections, as it has high selectivity, bioavailability, and safety . Herpes simplex virus (HSV) infections can be treated efficiently by the application of antiviral compounds. Herpes Simplex Virus (HSV), Molecular Detection, PCR, Spinal Fluid. Dosing and treatment of the specific clinical syndromes are described in .Auteur : Yu-Chen Jiang, Hui Feng, Yu-Chun Lin, Xiu-Rong Guo
Testing of herpes simplex virus for resistance to antiviral drugs
Manquant :
acyclovirCMV MANAGEMENT IN SOLID ORGAN TRANSPLANTATION
Higher frequencies of acyclovir .We show that genotypic antiviral resistance testing for HSV-1 can outperform phenotypic testing, which remains the clinical standard.Auteur : Amanda M Casto, Sean C Stout, Rangaraj Selvarangan, Alexandra F Freeman, Brandon D Newell, Erin D St.