Investigational new drug application fda
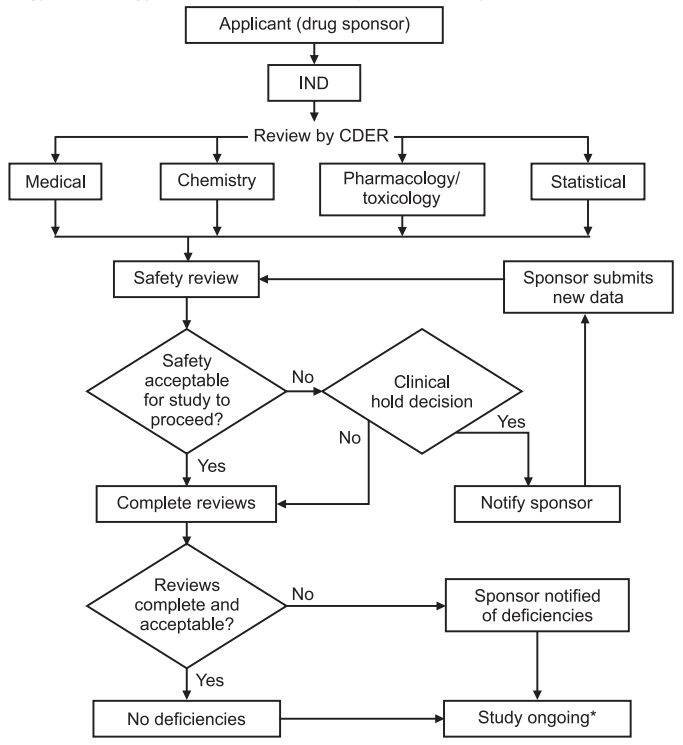
Investigational New Drug Applications (INDs) — Determining Whether Human Research Studies Can Be Conducted Without an IND . The resources for application . An IND must be authorized prior to interstate shipment and administration of any new drug or biological product that is not the subject of an .Wednesday, HOOKIPA Pharma Inc (NASDAQ:HOOK) received clearance from the FDA for its Investigational New Drug (IND) application for HB-700, a novel .The Food and Drug Administration (FDA)’s primary objective is to ensure safety.BMF-219 is a novel covalent menin inhibitor designed to regenerate insulin-producing beta cells with the aim to cure type 1 diabetes The FDA has cleared the .
IND Applications for Clinical Investigations: Overview
The initial IND submission to the FDA will provide the reviewers with the information necessary to conduct a thorough evaluation of the safety .IND Application Reporting: Safety Reports.
FORM FDA 3926
Individual Patient Expanded Access Investigational New Drug Application (IND) (Title 21, Code of Federal . This component of an IND application is expected to contain information about pharmacological and toxicological (laboratory animals or in vitro) studies on .Dockets Management Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852 All written comments should be identified with this document's docket number: FDA-1995-D-0251 .The United States Food and Drug Administration 's Investigational New Drug ( IND) program is the means by which a pharmaceutical company obtains permission to start .An Investigational New Drug Application (IND) is a request for authorization from the Food and Drug Administration (FDA) to administer an investigational drug or biological product to humans.This table provides links to information for investigators about submitting Investigational New Drug (IND) applications to FDA. DEPARTMENT OF HEALTH AND HUMAN SERVICES. Although in general the . Individual investigators may meet the FDA .
IND Application Procedures: Overview
All submissions with IND .
SOPP 8201: Administrative Processing of Clinical Holds for IND Applications
A 181 A signed .Investigator-Initiated IND Applications.23(a)(1), a sponsor-investigator’s initial IND submission must be accompanied by a 180 signed Form FDA 1571 Investigational New Drug Application (Form FDA 1571).IND Application Reporting: Overview. In the IND application . (a) A sponsor shall submit an IND to FDA if the sponsor intends .Initial submission of an IND for emergency use: During normal business hours (i.
What is an IND? Investigational New Drugs (INDs) are focused mainly on safety, and therefore, if applicable, preclinical toxicology, manufacturing, and pharmacology data are very important.

Food and Drug Administration is providing an at-a-glance summary of news from around the agency: On Thursday, the FDA posted a . FDA for VERVE-101 in Patients with Heterozygous Familial . quality and progress of an .Initial IND Application.GUIDANCE DOCUMENT.The purpose of this guidance is to assist sponsor-investigators in preparing and submitting complete investigational new drug applications (INDs) to the Center for Drug .NEW YORK and VIENNA, Austria, April 24, 2024 (GLOBE NEWSWIRE) — HOOKIPA Pharma Inc.米国食品医薬品局(FDA)による治験薬(ちけんやく、英: Investigational New Drug 、IND)プログラムは、医薬品の販売申請が承認される前に、製薬会社がヒト臨床試験を開始して実験薬を(通常は治験責任者に)出荷する許可を得るための制度である。 米国連邦規則21条312(21 C.Guidance for Industry Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well- Characterized, Therapeutic, Biotechnology-derived Products .
CMC Information for Human Gene Therapy IND Applications
For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).2 - Applicability.
Individual Patient Expanded Access Applications: Form FDA 3926
[CITE: 21CFR312.
Step 3: Clinical Research
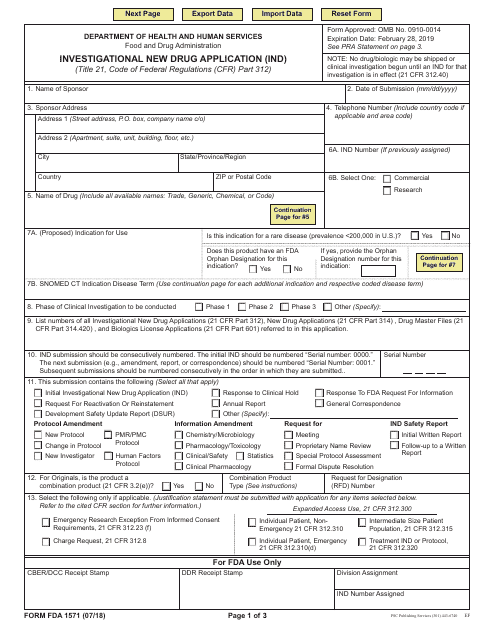
This guidance represents the Food and Drug Administration’s (FDA . Food and Drug Administration.April 19, 2024.important for the ongoing assessment of the safety of the drug under development.An Investigational New Drug Application (IND) is a request for authorization from the Food and Drug Administration (FDA) to administer an . is conducted early in phase 1, involves very limited human exposure, and.21 Phases of an investigation.Investigational New Drug (IND) Application is submitted to FDA if a drug (or biological product) not previously authorized for marketing in the US is intended to be used for the . The amendment should include the .IND Investigational New Drug Application ISE integrated summary of effectiveness ISS integrated summary of safety ITT intent to treat MedDRA Medical Dictionary for ., 8:00 AM – 4:30 PM, Monday to Friday), contact CBER’s Office of Communication, Outreach and Development of by . Drug developers, or sponsors, must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.Filing an IND requires completion of 3 sets of forms: 1 detailing the study (FDA Form 1571), 1 providing information about the investigator and study site (FDA Form 1572), and 1 .Subpart B - Investigational New Drug Application (IND) § 312. new information would further enhance the means by which FDA can (1) monitor the. CHAPTER I--FOOD AND DRUG ADMINISTRATION.A Good Review Practice, or GRP, is a “documented best practice” within CDER that discusses any aspect related to the process, format, content and/or management of a product review.The NDA application is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the U.

Verve Therapeutics Announces Clearance of Investigational New Drug Application by the U.
Types of Applications
NEW YORK and VIENNA, Austria, April 24, 2024 (GLOBE NEWSWIRE) -- HOOKIPA Pharma Inc.
IND Application Reporting: Overview
3 - Definitions and interpretations.Regulatory Affairs 101: Introduction to Investigational New Drug Applications and Clinical Trial Applications.
What is an IND?
The information on this page is current as of Dec 22, 2023.In September 2010, the Food and Drug Administration issued final regulations addressing the safety reporting requirements for investigational new drug applications (INDs) found in 21 CFR part 312 .7 - Promotion of investigational .
New Drug Application (NDA)
IND application sponsors are required to notify FDA in a written safety report of: any adverse experience associated with the use of the drug that is .An investigational new drug offered for import into the United States complies with the requirements of this part if it is subject to an IND that is in effect for it under § 312.A sponsor of an IND application is expected to submit a protocol amendment when a new investigator is added to carry out a previously submitted protocol.

Davy Chiodin, Erica M.We, FDA, are providing you, sponsors of human gene therapy Investigational New Drug Applications (INDs), recommendations regarding chemistry, manufacturing, and control (CMC) information submitted .21 - Phases of an investigation. INTRODUCTION This good review practice (GRP) document was prepared to assist FDA clinical reviewTemps de Lecture Estimé: 2 min
Investigator-Initiated Investigational New Drug (IND) Applications
Take advantage of the FDA’s accelerated programs, where applicable, to expedite . Please see the list below for available fiscal year reports on activities related to Investigational New Drug (IND) applications.20 Requirement for an IND.
Investigational New Drug Application
DEPARTMENT OF HEALTH AND HUMAN . An IND may be submitted for one or more phases of an investigation.
Initial IND Application
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) are health authority bodies that regulate the use of investigational drugs within the United States and the European Union, respectively.Studies using a drug that has not been approved by the Food and Drug Administration (FDA) or for indications not in the approved labeling may require filing an Investigational New Drug (IND) application with the FDA.
IND Application Reporting: Protocol Amendments
(NASDAQ: HOOK, ‘HOOKIPA’), a company developing a new .








