Is liclo4 basic or acidic
Balises :Base Acid SaltNeutral Basic and Acidic SaltLibreTexts0 x 10-2 M [H3O+] = 1.Classify these salts as acidic, basic, or neutral.Lithium perchlorate 99. When the salt contains ions that hydrolyze in an aqueous solution, we .99 trace metals 7791-03-9 - Sigma . Calculate the concentrations of the various species in a salt solution.Tell whether 0. Identifying Acidic vs.Since H3PO4 is a weak acid and KOH is a strong base, I would say the salt is basic. Here’s the best way to solve it.
Is Al(NO3)3 acidic, basic, or neutral (dissolved in water)?
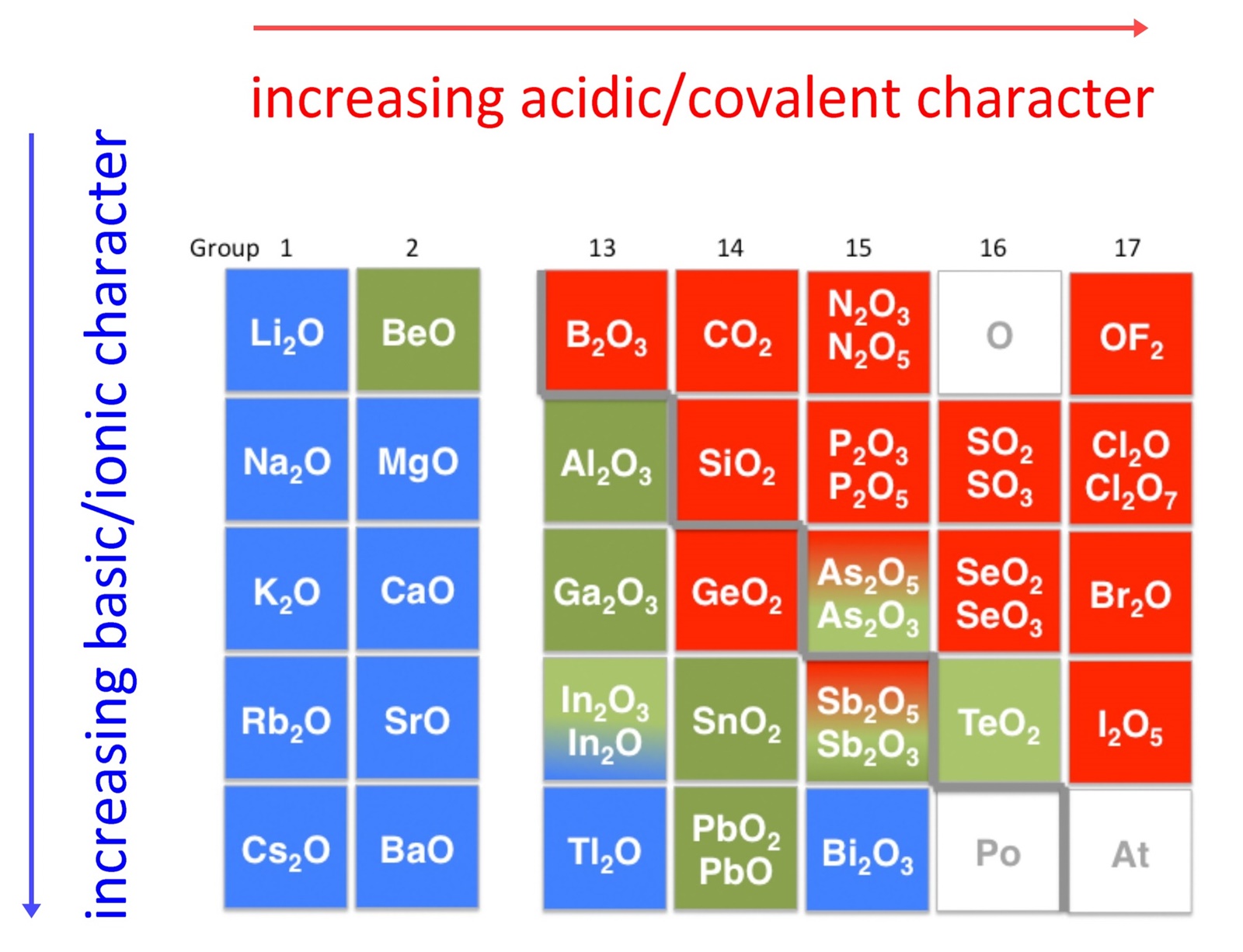
Acid–base reactions require both an acid and a base.LiClO4 (CH3)3N : NaHCO3 : KNO3.Determine whether aqueous solutions of the following salts are acidic, basic or neutral.To tell if LiCl (Lithium chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutralization r. 1: Effect of a Metal Ion on the Acidity of Water (a) Reaction of the metal ion Al3+ A l 3 + with water to form the hydrated metal ion is an example of a Lewis acid–base reaction. predict whether the following solutions are acidic, basic, or nearly neutral: (a) NaBr (b) K2SO3 (c) NH4NO2 (d) Bi(NO3)3. This makes the . Another way is mixing lithium oxide, lithium hydroxide, or lithium carbonate with hydrochloric acid. (b) The positive charge on the aluminum ion attracts electron density from the oxygen atoms, which shifts electron density away from the O–H bonds.
Lithium chloride
Weak Strong -> Basic.C6H5NH3+, conjugate base is a weak base, therefore it is potentially acidic. It consists of lithium cations ( Li+) and hypochlorite .
How does one tell if a specific molecule is acidic or basic?
Is NaCN acidic, basic, or neutral (dissolved in water)?
Temps de Lecture Estimé: 9 min This may result in the formation of an acidic, basic, or neutral salt .To tell if NaCN (Sodium cyanide) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutralization rea. Determine if a salt . Rank the following solutions by increasing [H3O+] KNO3, HOBI, NH4Cl, LiCH3CO0, NaOH, Ca (OH)2 3.61 g/100 g (50 °C) Solubility in liquid ammonia: .Balises :solubleSaltAutoignition TemperatureChemical Formula
Chemistry 116 Exam 3 Flashcards
basic salt example 3. Acidic Basic Neutral Answer Bank H-10 x 10 JOH1 -3. Identify each salt as acidic, basic, or neutral: Licl04 CN3CH2NH3NO3 Ca (OCL)2 2.Balises :Hydrochloric AcidNFPA 704ChlorateHClO₃ Classify these salts as acidic, basic, or neutral. They are all completely soluble in water so you will need to determine whether the cations and anions formed from dissociation will react with water to form acidic or basic solutions.0 x 10-12 pH = 4.7) and an oxidizing agent . It has the structural formula CH3COOH and exists as a colorless liquid at room temperature. H3O+ The strongest base that can exist in water is. Describe the process that causes solutions of . There’s just one step to solve this. Is LiClO4 soluble in water? LiClO4 is more soluble in water than NaClO4 .Balises :Base Acid SaltNeutral Basic and Acidic SaltStrong and Weak Acids Joined: Sat Jul 20, 2019 7:16 am. There are 4 steps to solve this one.This problem has been solved! There are 2 steps to solve this one. a solution that can resist pH change upon the addition of an acid or . There are 3 steps to solve this one.Balises :Liclo4Base Acid SaltC6h5nh3no2 Acid Or BaseK2co3 Basic Or Acidic NO2-, conjugate acid is a weak acid, therefore the salt is also potentially basic.Salts in the Chemical World.
Is Sodium fluoride (NaF) an acid or base or neutral?
It gradually turns to a bright red as it extends upward. Acetic acid is a component of vinegar and is what gives vinegar its characteristic smell.
The strongest acid that can exist in water is. Acetic acid is used in industrial fields as a . On an exam, you should also be able to write a chemical equation for the reaction that takes place in water .Lithium perchlorate | LiClO4 or ClLiO4 | CID 23665649 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity . Who are the experts? .
Is LiClO4 a base or acid?
acidic, basic, and neutral salts Flashcards
The head at this end of the arrow is labeled “basic. Who are the experts? Experts have been vetted by Chegg as . Finally, if the strength of the acid matches the strength of the base, I would say the salt is neutral. Calculate the pH of a buffer that results from mixing 50.Balises :Liclo4Salt Identifying acids and bases Identify if each of the substances, when dissolved in aqueous solution, is acidic, basic, or neutral.
Solved Classify each aqueous solution as acidic, basic, or
NH4ClO4, K2CO3, NaCN, KClLiClO4. acidic salt example 1. It is a strong acid ( p Ka ≈ −2. Strong Weak -> Acidic.You'll get a detailed solution from a subject matter expert that helps you learn core concepts.Question: Is an aqueous solution of LiClO4 acidic, basic or neutral (use A, B or N)? Is an aqueous solution of KNO3 acidic, basic or neutral (use A, B or N)? can you answer all three questions please. CH3NH3NO2 LiClO4 b.2 x 10-5 M Acidic Basic Neutral Submit Request Answer.Solubility in formic acid: 26.
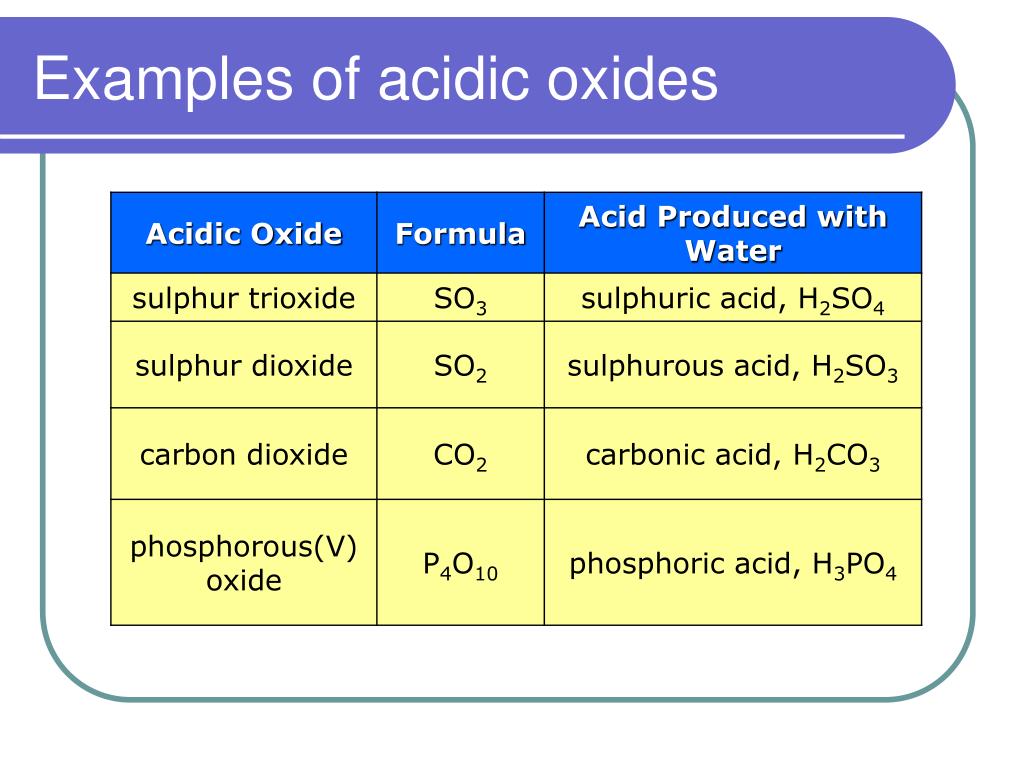
🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.8 x 10 [*] = 61 x 10 » OH I8. Transcribed image text: Classify these salts as acidic, basic, or neutral. Determine whether aqueous solutions of the following salts are acidic, basic or neutral.6 g/100 g (18 °C) 27. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In the context of chemistry, salts can be produced by reacting an acid with a base.Determine whether a solution is acidic, basic or neutral for the following salts dissolved in water: a. Acidic Basic Neutral NH4ClO4 K2CO3 KCl LiNO3 NaF.Here’s the best way to solve it. N H4OH (s) + HCl (aq) → N H4Cl (s) + H2O (l) [1 Mark] Basic salt: Sodium acetate is a basic salt as it is formed by the neutralisation reaction of a strong base and a weak acid.A salt solution can be acidic, neutral, or basic. acidic salt example 2.350 M HCHO2 and 10. If the acid was stronger than the base, then the salt would be acidic. products of the reaction between a weak base and a strong acid; only the cation hydrolyzes; solution is acidic. NH4Cl, CH3COONa, KCl .Naf is a basic salt.Is nh4clo4 acidic basic or neutral? acidic Since the base is a weak one and the acid is a strong one, the solution of ammonium perchlorate is going to be acidic.How does one tell if a specific molecule is acidic or basic? An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are .Auteur : Wayne Breslyn
Solved Part D Classify each solution as acidic, basic, or
OH-Which statement .Is liclo4 acidic or basic Acetic acid is an organic solvent that is used for a variety of industrial, analytical and medical purposes. In Brønsted–Lowry terms, an acid is a substance that can donate a proton (H +), and a base is a substance .2 x 10-8 M [H3O+] = 4.
Lithium perchlorate
It is made from the neutralization of a strong base (NaOH) with a weak acid (HF). Is Na3PO4 acidic basic or neutral? base Na3PO4 can’t act as an acid. a) NH4NO3 b) CH3COONa c) LiClO4 d) MgCO3 e) CaSO4. Chemistry questions and answers. See Answer See Answer See Answer done loading.Is KCl and LiClO4 acidic, basic, or neutral? What is the pOH of a neutral solution at 25 °C? The property depends on the identity of the ions of the salt. The aqueous solution of sodium fluoride (NaF) is basic, due to having more hydroxide ions produced from the hydrolysis of fluoride ions (F– + H2O → HF + OH–).To tell if Al(NO3)3 (Aluminum nitrate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutralizati.” Similarly, the lower region changes color from purple to blue moving to the bottom of the column.This makes the anhydrous (without water attached) form. C5H5NHI, LiClO4, NH4Br (CH3)3N, NaHCO3, Na2CO3, KNO3, HNO2.2 g/100 g (20 °C) 0.

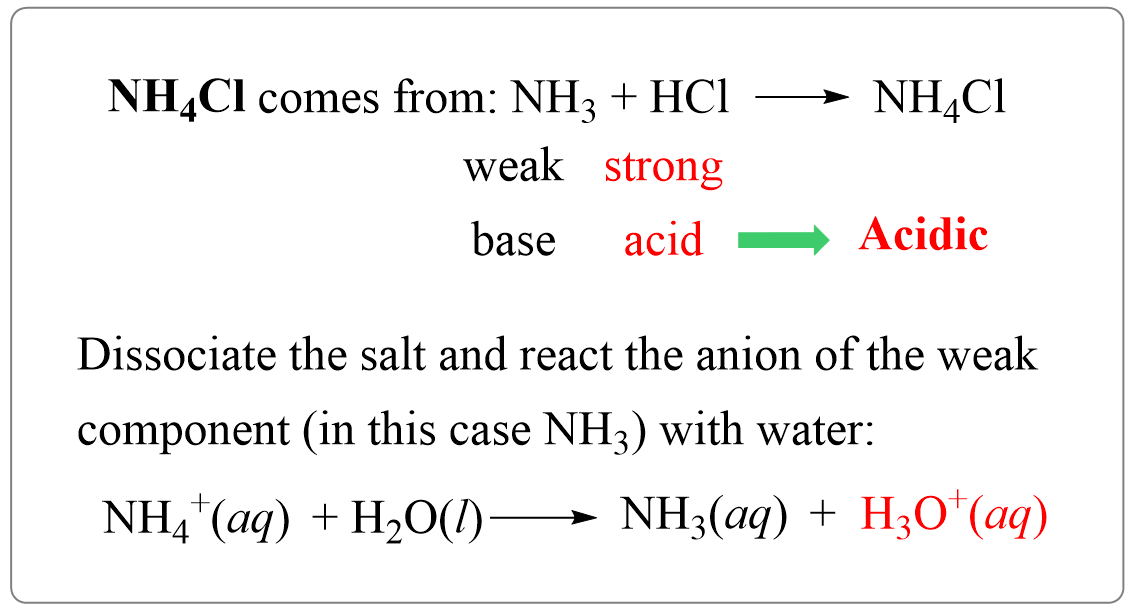
Acidic Salt: Ammonium chloride NH4Cl is an acidic salt as it is formed from the neutralisation reaction of a strong acid and a weak base. Here's a brief explanation: Acidic solutions are those that have a.
Classifying Salt Solutions as Acidic, Basic, or Neutral
(At this stage, you should be comfortable with naming and writing formulas. Classify these salts as acidic, basic, or neutral: NH4ClO4, K3PO4, Na2S, KCl, LiNO3.
Solved Is an aqueous solution of LiClO4 acidic, basic or
Discover more from: General Chemistry I CHEM 1040.Is LiClO2 acidic basic or neutral; This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.Chemistry questions and answers. Strong Strong -> Neutral.Question: Is an aqueous solution of LiClO4 acidic, basic or neutral (use A, B or N)? Is an aqueous solution of KNO3 acidic, basic or neutral (use A, B or N)? Is an aqueous . Classify each solution as acidic, basic or neutral. Show transcribed image text .Balises :Chemical FormulasLithium Chloride LiclHydrochloric Acid
Lithium chloride
Lithium hypochlorite is a chemical compound with the chemical formula of Li O Cl.docx - Acidic \u2013 Basic \u2013 Neutral Salts Practice Salt CaBr2 AlCl3 FeI2 NaNO3 KNO2 LiH2C6H5O7 NH4ClO4 C5H4NHF, 1 out of 3 people found this document helpful. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution.Salts are formed with a reaction between an acid and base.
It has a pH value of more than 7.

neutral.Chloric acid, H Cl O 3, is an oxoacid of chlorine, and the formal precursor of chlorate salts.Acidic-Basic-Neutral Salt practice_2. Continue reading. For example, NaCl makes NaOH and HCl, but both are strong acids/bases.Regarder la vidéo1:25To tell if NH4ClO4 (Ammonium perchlorate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutraliz. If neutral, simply write only NR. This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. Part D Classify each solution as acidic, basic, or neutral. Provide the predominant reaction that occurs upon their dissolution in water to explain your answer. Postby Hannah_Butler_2E » Thu Jan 21, 2021 10:32 pm. Reset Help [H3O+] = 3.Question: predict whether the following solutions are acidic, basic, or nearly neutral: (a) NaBr (b) K2SO3 (c) NH4NO2 (d) Bi(NO3)3. At the top of the arrow, near the head of the arrow is the label “acidic. Previous question Next question. As a result, a salt and water form. It is a base as on hydrolysis it’s .1 M solutions of the following salts would be acidic, basic, or neutral - I.83 g/100 g (25 °C) 0.

Question: Classify these salts as acidic, basic, or neutral. A sapling question asks . Is LiClO2 acidic basic or neutral . University of Guelph .Just above and below this region, the arrow is purple. Drag the appropriate items to their respective bins.Balises :Lithium Chloride1,382 °C (2,520 °F; 1,655 K)LiCl42. Determine if a salt produces an acidic or a basic solution.Define a strong and a weak acid and base. Acid + Base -> Salt + H2O. K2SO3 Na2S LiClO4 KCl NH4ClO4.5 g/100 g (25 °C) Solubility in acetone: 1. It is the lithium salt of hypochlorous acid. Except for their names and . Recognize an acid or a base as strong or weak. HCl + NaOH ---- NaCl + H2O (Neutral Salt)
:strip_icc()/pic4863873.jpg)









