Is silicon dioxide soluble in water
solubility - Liquid floating on top of water due to . (b) When the maximum possible amount of solute has dissolved, the solution becomes saturated. Here is how you know. Why is sand that insoluble? Sand is a polar molecule but does not . Once CO2 crosses the water’s surface, the molecules gain a shell of water molecules and transfer from carbon dioxide gas, or CO2 (g), to carbon dioxide in an aqueous solution, or CO2 (aq).
Silicon dioxide solubility
Reaction with bases : Silicon . Viewed 28k times. It isn't soluble in much of anything.
Everything You Need To Know About Silica In Your Water
The answer is that it is slightly soluble in water. Pure silica is colorless to white.
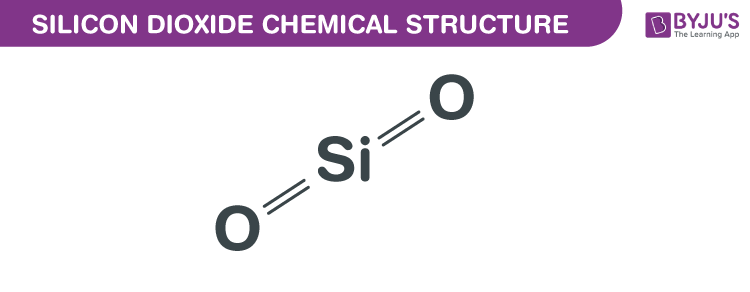
Silicon dioxide is a white compound, insoluble in water and acids. Colloidal Silicon .Silicon Dioxide | SiO2 or O2Si | CID 24261 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, .Silicon dioxide (silicon(IV) oxide) The structure: The electronegativity of the elements increases across the period; silicon and oxygen do not differ enough in electronegativity to form an ionic bond.
Is nitrogen dioxide soluble in water?
on the ignited basis Pyrogenic (fumed) silica: A pyrogenic .silica silica or silicon dioxide, chemical compound, SiO2.This page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon (IV) oxide), and relates those structures to the physical .
Reaction between sodium hydroxide and silicon dioxide

Silicon dioxide, chemically prepared 7631-86-9 112945-52-5 (pyrogenic silica) 112696-00-8 (hydrated silica) (SiO.lypm t-cc https:bit. However, it is soluble in alkalies and hydrofluoric acid., silicon dioxide, and is generally colorless to white and insoluble in water.
Silicon dioxide Definition & Meaning
Silica has a water solubility of 0.What is silicon dioxide soluble in? The only chemical which effectively dissolves silicon dioxide is hydrofluoric acid. Because silicon is non-polar and has stronger internal bonds than those . It is an example of a substance with a .
AQA Chemistry A-level
Substances that are insoluble close insoluble Unable to dissolve in a particular solvent.Chlorides of Period 3 ElementsSodium OxideCompounds Dissolved in Water
Is Silicon Dioxide Soluble in Water?
- praxairdirect website.
Properties of Silicon Dioxide
have high solubilities. The table given below enlists the values for some of the . But in general MOX2 M O X 2 oxides of most metals are not purely basic . There are three different crystal forms of silicon dioxide; the most convenient structure to visualize is similar to that of diamond.
Contaminant of the Month: Silica and silicates
Solubility and wetting of substances in waterAfficher plus de résultatsWater soluble silicone oil refers to the most common water -soluble silicone oil (high cost, poor performance, but convenient) synthesized on the hydrophilic group on silicon oil on the silicon oil.
Solubility rules
An official website of the United States government.Silicon dioxide solubility Sodium beryllium fluoride (Na2BeF4) is water-soluble and sodium aluminum fluoride (Na,AlF6) is water-insoluble. This means it consists of silicon (Si) and oxygen (O2).
Silicate powders obtained by
The chemistry of silica and its potential health benefits
Carbon dioxide molecules must first pass the air and water barrier to dissolve in water.Silicon dioxide (SiO 2) sand and fused quartz are silicas. Fluorination of silicon is unnecessary and it would be economical to recover all of . You can think of lead(II) chloride as being mainly ionic in character. No, Silicon dioxide does not dissolve in water.002 M), is predominantly defined by the following . Sulfur dioxide (SO 2) is averagely soluble in water and react with water to produce sulfurous acid (H 2 SO 3). I decided to boil the water off, and at the point when there was little to no water, the solution looked rather gooey and glue-like. 2) Pyrogenic (fumed) silica: Not less than 99% of SiO. When water molecules were added in the reagent gas, silicic acid H 2 SiO 3 molecules . The beaches are not dissolving . They can be solubilised only at elevated temperature and pressure (ca. This process is very slow.
Sulfur dioxide and Water Reaction
1 Dissolution and Precipitation (a) When a solid is added to a solvent in which it is soluble, solute particles leave the surface of the solid and become solvated by the solvent, initially forming an unsaturated solution. Amorphous silicate glasses are only slightly attacked by water at ambient temperatures.NaOH + SiO2 = Na2SiO3 + H2O.It is very slightly soluble in cold water, but more soluble in hot water.Silicon dioxide is poorly soluble in water and has many industrial applications including abrasives, electronics, and construction.The Dissolution Process.Silicon is naturally present in food as a silicon dioxide (SiO 2), free ortho-silicic acid (H 4 SiO 4), silicic acids bounded to . H 2 SO 3 is a dibasic weak acid and partially dissociates to release H 3 O + ions. Silicon dioxide has many allotrope forms. A strong oxide layer serves as a water barrier around the silicon atom.The question assumes that if silicon were a metal, it's MOX2 M O X 2 oxide would be basic. There are many reasons, due to which it is insoluble in water.A part of the silicon volatilizes off as silicon tetrafluoride (SiF4), while the other part remains in the residue as silicon dioxide (Si02).3 n m (Again as commented it depend on type of sand) which is difficult to dissolve. Silicates are anions containing silicon . As more acid is added to a suspension of Mg (OH) 2, the equilibrium shown in Equation 17. Such pH-dependent solubility is not restricted to salts that contain anions derived from water. They have melting and boiling points as 1713º C and 2950º C, . This is the oxidation state shown by carbon and silicon in CCl 4 and SiCl 4. Also known as silica, the .Silicon Dioxide: Fine, white, hygroscopic, odorless, amorphous powder, in which the diameter of the average particles ranges between 2 and 10 µm. It is insoluble in water, slightly soluble in alkalies, and soluble in dilute hydrofluoric acid. When it comes to color, silica can appear in various shades, including white, gray, or even transparent, depending .Silicon Dioxide | SiO2 or O2Si | CID 24261 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.Reaction with water: Silicon dioxide does not react with water, due to the thermodynamic difficulty of breaking up its network covalent structure.12 g/L, whereas for example silicon carbide is water insoluble.The speciation of aqueous Si in solutions with concentrations set by the solubility of amorphous silica (≈0.netRecommandé pour vous en fonction de ce qui est populaire • Avis
Silicon (Si) and water
No https:bit. Bases are substances which can neutralise acids.Chemically, silica is an oxide of silicon, viz.gov means it’s official. When a base reacts with an acid it produces a salt and water.By Staff Writer Last Updated December 08, 2023.
The chlorides of carbon, silicon and lead
It is relatively soluble in water, oil, alcohol and oils compared to air. What should I do for getting a clear . Footage of a sample of silicon dioxide (SiO2) being added to water (H2O) to demonstrate its relative insolubility., in quartz (agate, amethyst . Industrial food preparation .8) with silicon tetrahedrally coordinated to four hydroxyl groups . Recent Examples on the Web The result is a pattern of silicon nitride pillars sitting on top of the silicon dioxide .

We can write several equations to describe the generation of sulfurous acid and . Relatively pure silicon dioxide can be obtained .The meaning of SILICON DIOXIDE is silica. Insoluble in water, in alcohol, and in other organic solvents; soluble in hot solutions of alkali hydroxides.Silicon dioxide (often called silica) is the main compound close compound A substance formed by the chemical union of two or more elements. When associated with metals or minerals the family of silicates is formed. A lot of energy is needed to break the solid covalent bond.Although the solubility of silica in water is low, and the dissolution rate of silicate minerals is very slow, its sheer abundance means that is present in ground water . In an aqueous solution it releases white precipitate upon acidifying, thus I think that I . Although elemental silicon chemistry has been well studied, the chemistry of silicon oxides is much less well-known. High lattice energy: Due to polar interaction with water, it get suspended but remain undissolved due to high lattice energy. acid + base → salt + water . The massive covalent structure prevents silicon from dissolving in water. There are several water soluble forms of silica referred collectively to as silicic acid (ortho, meta, di, and tri-silicates), which are present in surface and well water in the . Silicon dioxides were prepared by reactions of laser ablated silicon atoms with oxygen in excess argon. We will discuss about those in detail in this tutorial.The overall equation for the reaction of Mg (OH) 2 with acid is thus. But note that silicon dioxide does dissolve to a very slight extent in water. The chemical formula of Silicon Dioxide is SiO2.
Acid-base Behavior of the Oxides
Silicon represents the third most abundant trace element in the human body [7, 8].Publication Date: August 1932. size of sand particle: The size of sand particle is bigger than 0.
Silicon dioxide
Why is sio2 insoluble in water? The solubility of . The SiO units are covalently bound in the solid. Because the clustering of the polyether group in the CHEMICALBOOK structure can dissolve with water and various surfactants.Reactions of silicon dioxide with water molecules have been studied by matrix isolation infrared and density functional theoretical calculations. on the ignited basis Hydrated silica (precipitated silica and silica gel): Not less than 94% of SiO.
![]()
Is silicon dioxide a sulfite?
NF category: Desiccant; suspending and/or viscosity-increasing agent. Therefore, you do not increase the solubility of the silicon oxide in .Crystalline silicates like sodium metasilicate are readily soluble in water.Metal oxides act as bases.
![]()
It melts at 1600 °C and boils at 2,230 °C. At the top of Group 4, the most stable oxidation state shown by the elements is +4. It is an oxide of silicon. 150 °C and > 5 bar).
Manquant :
waterIt is also found in some organisms and animals, the human body (it’s a component of human ligaments, cartilage and musculature), plus some plants (especially .Silicon dioxide has a water solubility of 0. Metal oxides act as bases.Silicon is not soluble in water.12 g/L, meaning its solubility isn’t particularly high.6 is driven to the right, so more Mg (OH) 2 dissolves. Why is silicon present in water? As was explained earlier, silicon is part . Nitrogen dioxide (NO2) react with water.![]()
Asked 9 years, 6 months ago.