Is water evaporation exothermic
However, as the pressure changes, the boiling point also changes. We all know that water evaporates when the temperature climbs, but researchers have just shown that there's another factor at play.comEvaporation: Definition, Meaning, Water Cycle, Endo or . Describe how heat is transferred in endothermic and exothermic reactions. An exothermic reaction gives off heat energy.The teacher replies: “That’s because you were given two different salts.Temps de Lecture Estimé: 5 min
Evaporation
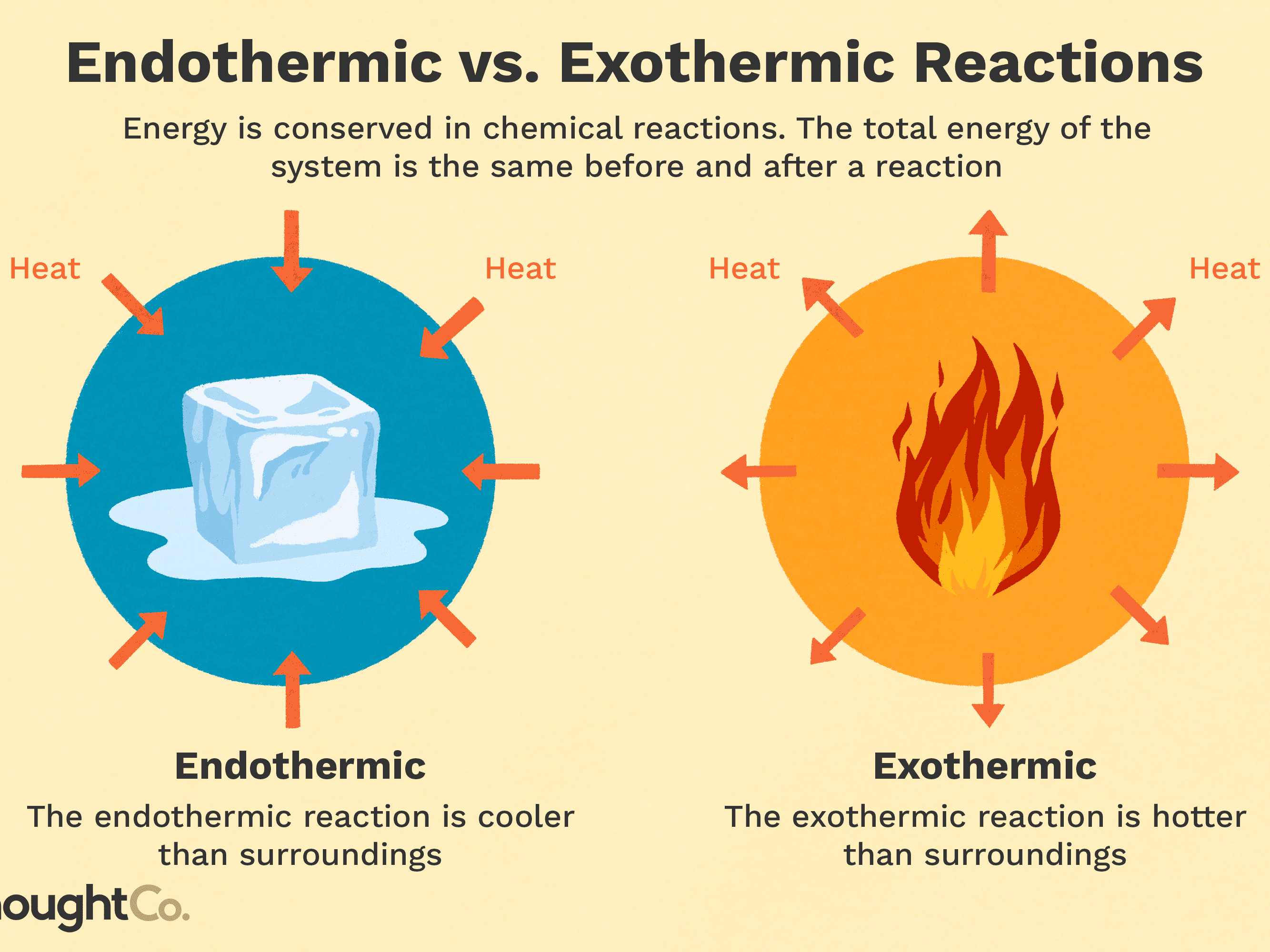
Expand/collapse global location. The reaction will happen at a . It can be easily visualized when rain puddles “disappear” on a hot day or when wet clothes dry in the sun.Experiment 6 Prelab Quiz Flashcards | Quizletquizlet.Evaporation, is a physical process whereby water in li An Exothermic Reaction will have an Exothermic change, whereby energy (In the form of heat) is release/given out to the surrounding.The surroundings is everything in the universe that is not part of the system. In an exothermic reaction, heat is released (considered a product) and the energy of the system decreases (Δ H is negative).Water boiling: The process of boiling water is endothermic. It is also how liquid water enters the atmosphere as water .comList of Phase Changes Between States of Matter - ThoughtCothoughtco. A puddle of water left undisturbed eventually disappears.“Yes! A reaction that releases the same amount of energy as is put in is called an isothermal reaction. It can occur at any temperature below the . The amount and speed of evaporation are determined by external factors like heat, humidity, airflow, and container type.The evaporation of water is endothermic. As with the melting point of a solid, the temperature of a boiling liquid remains constant and the input of energy goes into changing the state. Join / Login >> Class 11 >> Chemistry >> Thermodynamics >> Internal Energy as a State of System >> Evaporation of water is: | Chemistry Que. Since Energy is Absorbed to break the bonds before it can become a gas ,that makes Evaporation an Endothermic Process .Heat from the sun, or solar energy, powers the evaporation process. This process is crucial in regulating the earth’s temperature and . When energy is transferred as heat from the surroundings to the system, Δ H is negative.Think again, says MIT.
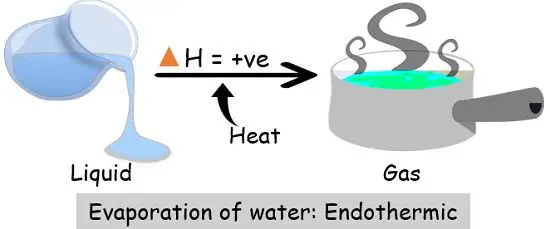
Since the liquid water molecules must absorb energy (heat) to convert to water vapor (gas) molecules, evaporation is an endothermic process.
How is evaporation of water endothermic?
Energy diagrams visually represent potential energy changes during a reaction, providing .By Serm Murmson. Hence, boiling water is an endothermic . The categorization of a reaction as endo- or exothermic depends on the net heat transfer.4: Evaporation and . 2: Cooling Curve for Water. You visited us 0 times! Enjoying our articles? Unlock Full Access! Question.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Heat of Vaporization
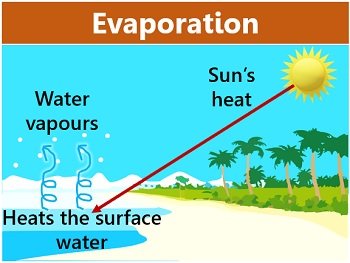
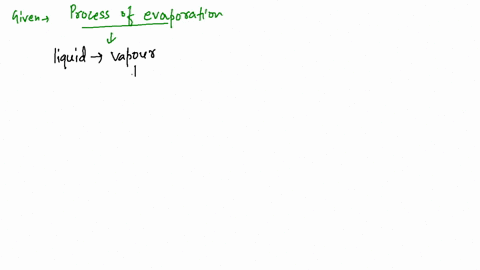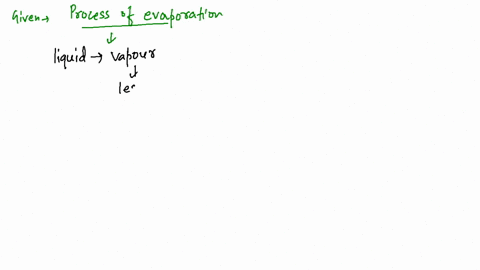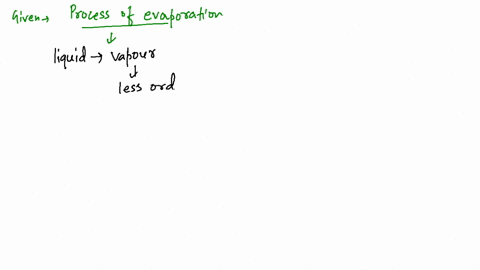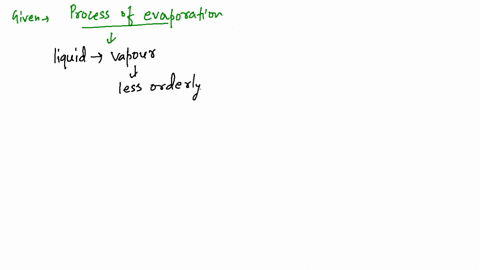
A combustion reaction is exothermic. Evaporation is a very important part of the water cycle.Is Evaporation Endothermic or Exothermic? | Jacks Of Sciencejacksofscience. Whereas a reaction is considered exothermic if heat or light are emitted as byproducts.And such a reaction is referred to as an exothermic reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively.Our bodies use this same phenomenon to maintain a constant temperature: we perspire continuously, even when at rest, losing about 600 mL of water daily by evaporation from . Tiny droplets of water in the atmosphere accumulate to form clouds, which can return the .
Explaining Why Condensation Is Exothermic
In an exothermic .8: Exothermic and Endothermic Processes is shared under a CC . Study the evaporation process of water from Earth's surface into the sky where water . Conversely, the . Concluding Remarks. Question: Which statements are true? Δ𝐻ΔH for an exothermic reaction is positive.Heat of Vaporization and Condensation.Exothermic: when heat from a system is given off into its surroundings, due to the increase in heat in a reaction Heat of Vaporization: the . On the other hand, evaporation is not dependent on a specific temperature.Regarder la vidéo8:00If ΔH is positive, the process absorbs heat from the surroundings and is said to be endothermic. Since the liquid water molecules must absorb energy (heat) to convert to water vapor (gas) molecules, evaporation is an endothermic process. Is water evaporating from your skin endothermic or exothermic? Explanation: Sweating reduces skin temperature because the sweat that is secreted to the skin evaporates, which is an .In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. This is mostly because heat must be applied in order for water to boil. Determine whether a reaction is endothermic or . Energy from the Sun causes water to evaporate.Evaporation happens when a liquid turns into a gas.

What kind of process (endothermic or exothermic) is evaporation? Endothermic because it draws heat from the surrounding, rather than giving it off. See an expert-written answer! We have an expert-written solution to this problem! A calorimeter . When energy is transferred to the surroundings, this is called an exothermic reaction and usually . Linking this to solid, liquid and gas states, when you vaporise a substance (turn it into a gas) you are promoting the particles in the .When water is heated, it e vaporates.
Q: Is the evaporation of water endothermic or exothermic?
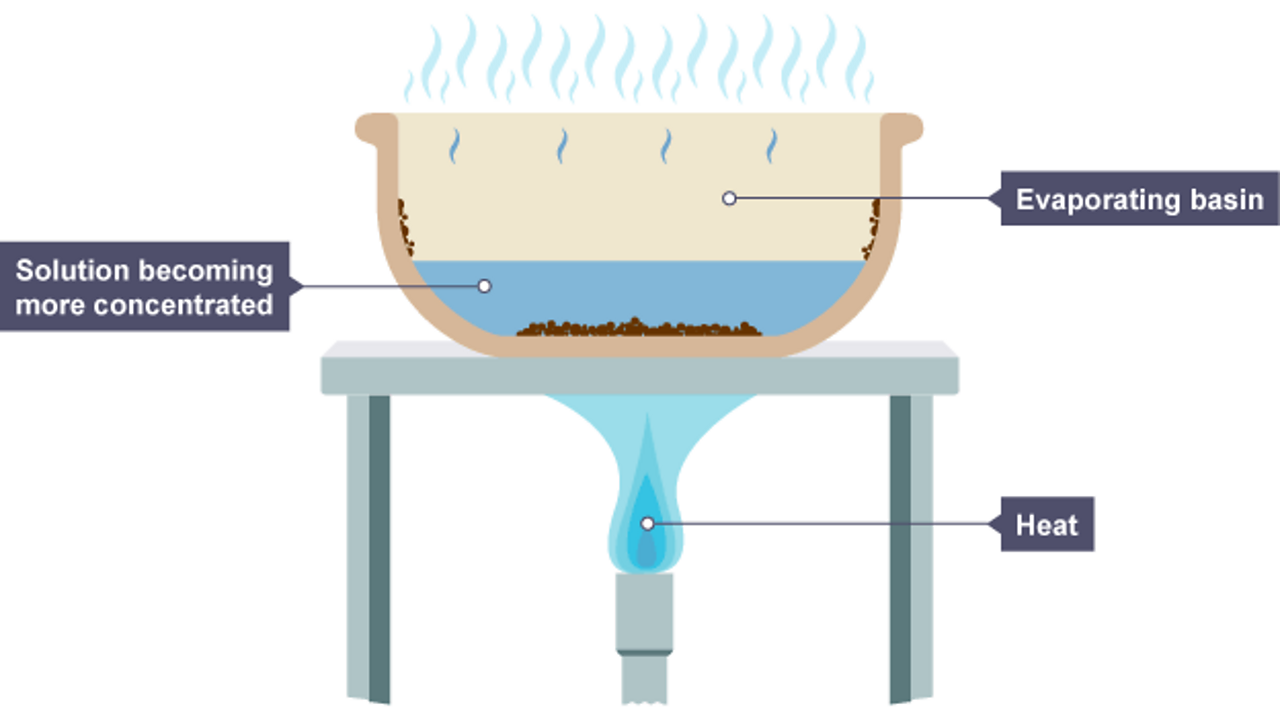
Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature.orgThe Process of Evaporation - National Geographic Societynationalgeographic. In these examples, the liquid water is not actually vanishing—it is evaporating into a gas, called water vapor. Combustion is an example of an exothermic reaction. Alongside condensation .Exothermic and Endothermic Reactions. At higher altitudes, where the atmospheric pressure is lower, water boils at a lower temperature. The liquid molecules escape into the gas phase, becoming water vapor. Evaporation happens on a global scale. Since the liquid water molecules must absorb energy (heat) to convert to water vapor (gas) . It’s a natural process that takes place both in nature and in artificial systems. an exothermic change. Which of the following are exothermic processes? (i) .comEndothermic vs Exothermic Reactions - Diffendiffen. Endothermic chemical reactions are those in which the reactants take in heat energy from their environment to create products. Condensation is the process by which water vapor turns into liquid water. An example of this is water boiling. We can use the law of conservation of energy to determine how that energy is transferred between a system and its surroundings.Once the exothermic oxidation generates sufficient energy to overcome heat losses (i.The heat may be supplied by a purely physical heat source (e. In the applied smoldering of HMWs, MC acts as an energy sink, in which a high MC can significantly impact reaction propagation .comIs condensing water vapor an exothermic process or an .q=mc ΔT (energy of a temperature change within a phase) q=n ΔH transition (energy of a phase transition) It needs to be realized that if you add heat, you move to the right, and if you remove heat, you move to the left. This typically . As one needs to supply heat energy to boil water, this chemical reaction is considered an endothermic reaction. Note, Increasing heat moves to the left, removing heat moves to the right.Is the evaporation of water endothermic or exothermic? Flexi Says: Evaporation changes liquid water to water vapor.Define endothermic and exothermic reactions. The molar heat of vaporization \ (\left ( \Delta H_\text {vap} \right .

A reaction or change is exothermic if heat is released by the system into the surroundings. As the water falls, some of its potential energy is converted into kinetic energy. Because the surroundings are gaining heat from the system, the .
Experiment 6 Prelab Quiz Flashcards
ΔH for an endothermic reaction is positive When energy is transferred as heat from the system to the surroundings, ΔH is negative.Afficher plus de résultats Energy is absorbed in the process of converting a liquid at its boiling point into a gas.Evaporation changes liquid water to water vapor. an endothermic change.The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor. When energy is transferred as heat from the system to the surroundings, Δ H is negative., a positive energy balance), smoldering has the potential to be self-sustained and can propagate without external energy input.
Endothermic and exothermic processes (video)
The evaporation of water is an exothermic process. Chemical reactions can result in energy being released (exothermic) or energy being absorbed (endothermic).Water evaporation and condensation are important mechanisms in the energy conservation of smoldering. Here the heat energy is provided, which breaks the bond of molecules/atoms in the liquid state. Let me first reveal the identity of your salts: Salt A is ammonium nitrate ( NH 4 NO 3 ) and Salt B is calcium chloride ( Ca Cl 2 ).Introductory Chemistry.A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Evaporation is the opposite of .The evaporation of water is an exothermic process.4: Evaporation and Condensation.
Q: Is evaporation endothermic or exothermic in nature?
Manquant :
water evaporationIf ΔH is negative, the process releases heat to the surroundings and is said to be .Evaporation happens when a liquid substance becomes a gas.Explain It to A Child
What Phase Changes Are Exothermic & Endothermic?
In order for the hydrogen bonds to be broken in water (which is required for water to evaporate), an input of energy is needed.This process is called condensation and releases energy (exothermic). a sufficient amount of a red-hot substance that is dropped into the water); the heat may also be .
Is water evaporating an exothermic process?
Vaporization is the process .
Click here:point_up_2:to get an answer to your question :writing_hand:which of the following are exothermic processesi evaporation of wateriidilution of an acid h2so4iiireaction of.
Δ H for an exothermic reaction is positive. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water.evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at which it boils; in particular, the process by which liquid water enters the atmosphere as water vapour in the water cycle.When liquid or water changes into gas or vapors, the process is termed evaporation. 2: (a) Water that is higher in elevation, for example, at the top of Victoria Falls, has a higher potential energy than water at a lower elevation. It is an essential .
Is Evaporation Endothermic or Exothermic?
Exothermic processes ( graph B) are the opposite of endothermic processes - energy is released by a system / reactants and the particles involved have a lower energy after the process than before.
Is water boiling exothermic or endothermic?
Why is heat released or absorbed in a chemical .