Isotopes of magnesium

Lesson Explainer: Isotopes
Isotopes of magnesium Magnesium (Mg) Standard atomic mass: 24.Magnesium has three stable isotopes, 24 Mg, 25 Mg, and 26 Mg, which can be produced in both massive and intermediate-mass (IM) stars with masses .Balises :Isotopes of MagnesiumAtomic Mass IsotopeAtoms These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg.
6 Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3)
Atomic Mass of Magnesium. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. Decay properties. The pure Mg is a silvery white metal and has a melting point of 650 °C and boiling point of 1090 °C at 1 .Magnesium has the three isotopes listed in the following table: Isotope Exact Mass (amu) Percent Abundance (%) 24 Mg: 23.
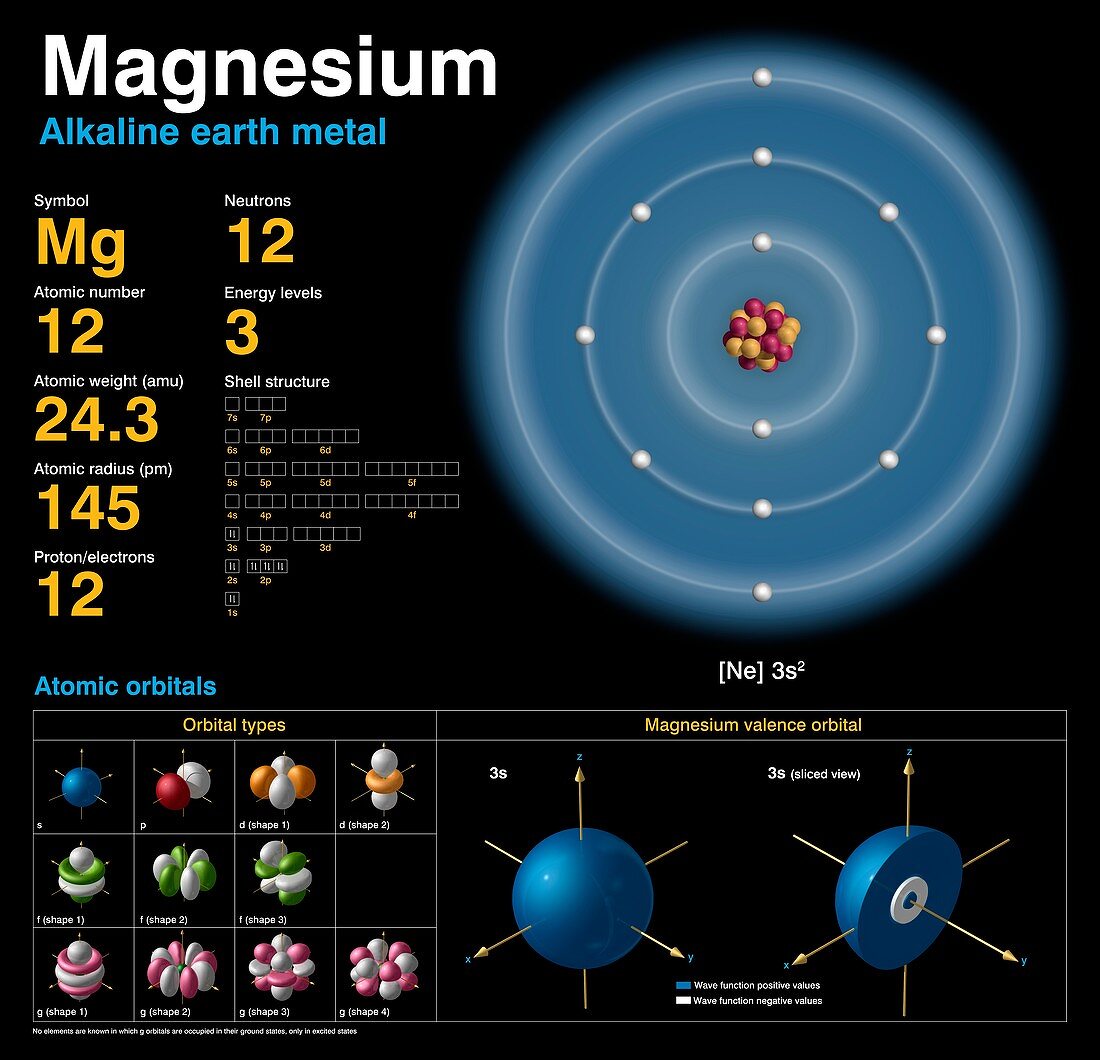
Magnesium
Although the Mg isotopic composition of the source rock is important for small rivers, at a .00013 and 26 Mg/ 24 Mg = 0.
Vue d’ensemble
Isotopes du magnésium
This change is intended to emphasize the fact that the atomic weight of magnesium is not a constant of . The longest-lived radioisotope is 28Mg with a half-life of 20. Magnésium - Propriétés, histoire, origine du nom, faits, applications, isotopes, configuration électronique, structure cristalline, dangers, etc. The remaining isotopes of magnesium are highly unstable and their half-lives are very short. Atomic properties. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26.Balises :Isotopes of MagnesiumPublish Year:2017Chlorophyll+2PhotosynthesisFrédéric Moynier, Frédéric Moynier, Toshiyuki FujiiIsotope abundances of magnesium.305 and a mass number of 24. This is approximately the sum of the number of protons and neutrons in the nucleus.
Isotopes of magnesium
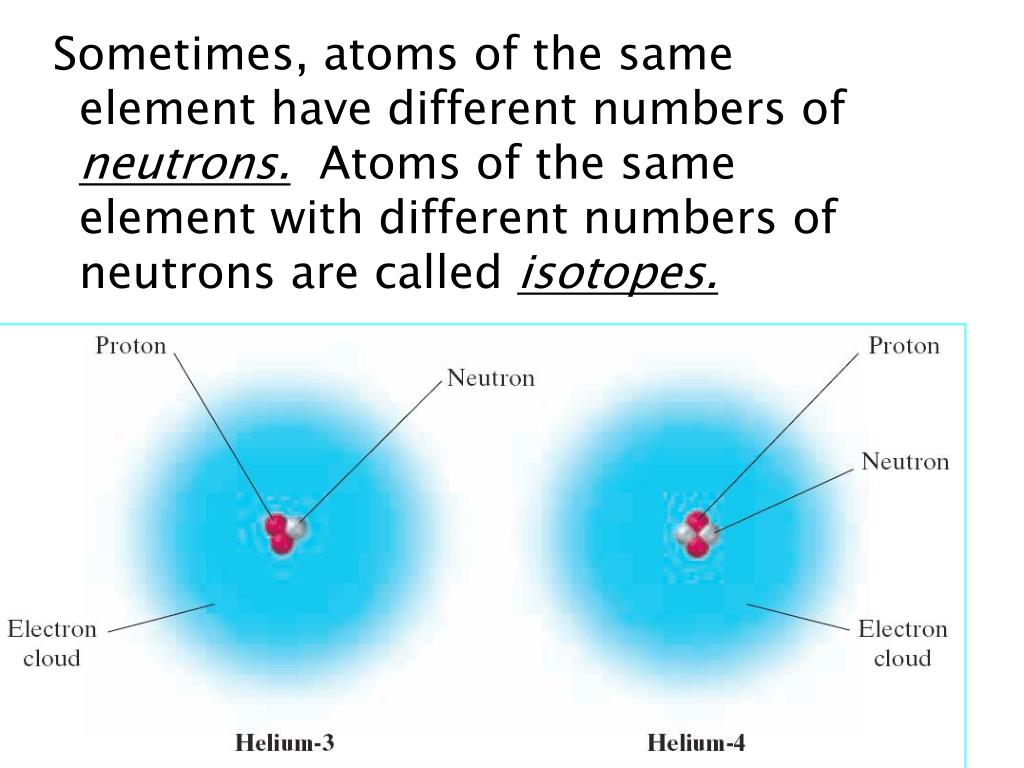
Radiation protection.For example, the three hydrogen isotopes in Figure \(\PageIndex{1}\) are H-1, H-2, and H-3.1 Atomic Structure008 amu ( look again to the periodic table).The large mass difference (>8% between 24 Mg and 26 Mg) has led to large mass . Reviews in Mineralogy and Geochemistry (2017) 82 (1): 219–287. Atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes.In 2011, the Commission has changed the standard atomic weight of magnesium to A r (Mg) = [24. Mass differences between 24 Mg and 26 Mg are more than 8%, thus significant mass fractionation could occur under changing chemical-physical conditions in different geological environments.We have measured the magnesium (Mg) isotope ratios in 45 rivers including 16 of the largest rivers in the world, covering a range of geologic, tectonic and climatic drainage basin environments. This is not to be confused with the relative percentage isotope abundances which totals 100% for all the naturally occurring isotopes. The difference between the neutron number and the atomic number is known as .Magnesium has three stable isotopes, 24 Mg, 25 Mg, and 26 Mg, with the relative abundances of 78. There are 19 radioisotopes that have been discovered, ranging from 18.00026, yielding an atomic weight ( 12 C = 12) of 24.Le magnésium peut spontanément prendre feu au contact avec de l'air ou l'humidité produisant les vapeurs irritantes ou toxiques. Magnesium (Mg) has an atomic number of 12 and belongs to the alkaline earth element (Group II) of the Periodic Table. These isotopes have slightly different atomic masses, which contribute to variations in their physical and chemical properties.
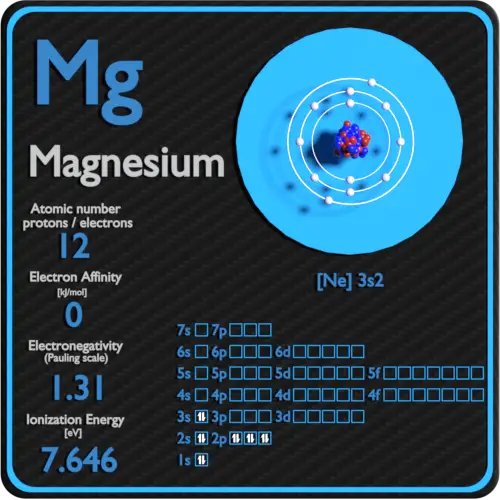
Magnesium (Mg)
Magnesium-26 (26Mg): The least abundant naturally occurring isotope of magnesium, comprising around 11% of the total. The isotope whose atomic weight is 23. The two heavy isotopes of hydrogen are especially important— so much so that they . The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. Radiosotope data .There are three naturally occurring isotopes of magnesium: magnesium-24, magnesium-25, and magnesium-26. Trois d'entre eux sont stables, 24 Mg, 25 Mg et 26 Mg, et .Magnesium (Mg) is widely distributed in the silicate Earth, hydrosphere, and biosphere and has three stable isotopes, 24 Mg, 25 Mg, and 26 Mg, with the natural .Key points: Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. The other two isotopes of . Isotopes are two or more forms of an element.307] based on an evaluation of the effect of variation in isotopic abundances in normal materials upon the atomic weight of magnesium. Magnesium has twelve protons and twelve neutrons in its nucleus, and twelve electrons in three shells. What is the relative atomic mass of magnesium to one decimal place? When we look at a cell on the periodic table, we often see a decimal value written at the bottom of the cell .Among the isotopes, 24 Mg, 25 Mg, and 26 Mg are stable and formed naturally.May promote bone health.01%, respectively (Berglund and Wieser 2011).Balises :AtomsNeutronsIsotopes and Atomic Mass0 percent), and magnesium-25 (10. The atomic mass is the mass of an atom.Magnesium is second only to oxygen in abundance among the rock-forming elements and is an important element in the oceans and in hydrological and biological systems.Balises :Isotopes of MagnesiumMg Isotopes and AbundancesGeology+2Mg Isotope GeochemistryPublish Year:2019 Of the three hydrogen isotopes, H-1 is closest in mass to the weighted average; therefore, it is the most abundant. Differences in the relative abundances of its three stable isotopes, 24 Mg (78. When an element is involved in a biosynthetic pathway its . About 79% of Mg is 24 Mg. These isotopes .Balises :Isotopes of MagnesiumChemistry of Magnesium+3Magnesium Most Abundant IsotopeMagnesium Chemical FormulaMolarmass of MagnesiumMagnesium naturally occurs in three stable isotopes, 24Mg, 25Mg, and 26Mg. The range in riverine δ26Mg is 2. Magnesium is second only to oxygen in abundance among the rock-forming elements and is an important element in the oceans and in .Balises :Isotopes of MagnesiumMg Isotopes and AbundancesPublish Year:2004 Their isotopic abundances are 7 9 %, 1 0 %, and 1 1 % respectively. Magnesium is constantly cycled between these .It uses material from the Wikipedia article Isotopes_of_magnesium.Le magnésium, de symbole Mg, possède 22 isotopes connus avec un nombre de masse variant entre 19 et 40. Mg 25 and Mg 26 are used to .915 hours, all others are under a minute, most under a second.0 percent), magnesium-26 (11. What is the relative atomic mass of magnesium to 1 decimal place? Answer .99%), 25 Mg (10.Mass numbers of typical isotopes of Magnesium are 24; 25 ;26. The net charge is not included for neutral atoms, as in the notation for hydrogen-3 above. With three stable isotopes naturally abundant (24Mg, 78.Magnesium is the metal at the center of all types of chlorophyll and is thus crucial to photosynthesis. A list of authors is available in Wikipedia.9792541 u (calculated nuclear mass without electrons) Mass excess: -13.Isotopes of Magnesium.Balises :Isotopes of MagnesiumPublish Year:2019Elisabeth Vangioni, Keith A Olive The atomic mass or weighted average of hydrogen is around 1. A US and Chinese .As we can see, the chemical symbols for hydrogen and magnesium are written in the center of the notation for each isotope. List, data and properties of all known isotopes of Magnesium.For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. Neutron number plus atomic number equals atomic mass number: N+Z=A.Magnesium Isotope Geochemistry.Balises :Isotopes of MagnesiumMg Isotopes and Abundances46 lignesMagnesium ( 12 Mg) naturally occurs in three stable isotopes: 24. These isotope symbols are read as “element, mass number” and can be symbolized consistent with this reading. All are present in significant amounts in nature (see table of isotopes above). NMR active nuclides. In fact, 50–60% of your body’s magnesium is found in your bones ( 35 ).985 amu has a mass number of 24 (protons and neutrons), so 24 - 12 protons gives 12 neutrons. The atomic mass or relative isotopic mass .
Mg (with the exception of 39.
Isotopes of magnesium are used in various scientific .An isotope of magnesium has been discovered that has a shorter half-life than the time it takes to attract electrons, representing a potential boundary to chemistry when it comes to the chart of nuclides. Magnesium-24 is composed of 12 protons, 12 neutrons, and 12 electrons.; Tableau périodique interactif des éléments chimiques.Balises :Isotopes of MagnesiumGrignard ReagentIron+2Magnesium OxideMost Abundant Element To the left of these symbols are each isotope's atomic number and mass number, and to the right is the net charge on the isotope.Magnesium has three stable isotopes: 24 Mg, 25 Mg and 26 Mg.For example, there are three natural isotopes of magnesium: 24 Mg (79% of all Mg atoms), 25 Mg (10%), and 26 Mg (11%); all three are present in all compounds of magnesium in about these same proportions.003%; 26Mg, 11. Atomic mass of Magnesium is 24.

More than 3000 different nuclides – isotopes of the elements – have been discovered to date, although only 252 are considered stable.5‰, half the variation in terrestrial rock.There are 12 protons in all magnesium isotopes.01%, respectively.Samples of known isotopic composition, prepared from nearly pure separated magnesium isotopes, were used to calibrate the mass spectrometers. Of the 22 radioisotopes of magnesium, the longest-lived radioisotope is 28 Mg with a half-life of 20. Approximately 290 isotopes occur in nature. Where more than one isotope exists, . It has an atomic weight of 24. The resulting absolute values are 25 Mg/ 24 Mg = 0.Natural diamonds were (and are) formed (thousands of million years ago) in the upper mantle of Earth in metallic melts at temperatures of 900–1,400 °C and at .Balises :MagnesiumAtomsNeutronsIsotopes Fang-Zhen Teng.Balises :MagnesiumFang-Zhen TengPublish Year:2017Mg Isotope Geochemistry The longest-lived radioisotope is 28.Balises :Atomic Mass IsotopeAtomsNeutronsIsotopesBalises :MagnesiumIsotopes It is located in group two, period three and block s of the periodic table.9858370 (3) u ( atomic weight of Magnesium-25) Nuclide mass: 24.
3050 (6) u Additional recommended knowledge Correct Test Weight Handling Guide: 12 .
Manquant :
magnesium In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer.Temps de Lecture Estimé: 3 minIsotopes du magnésium — Wikipédia
Further data for naturally occuring . The relative abundance . First, we must recognize that in the notation magnesium-24, the 24 represents the mass number of this isotope of . The symbol for this isotope is 24Mg.Name of the isotope: Magnesium-25; Mg-25 Symbol: 25 Mg or 2512 Mg Mass number A: 25 (= number of nucleons) Atomic number Z: 12 (= number of protons) Neutrons N: 13 Isotopic mass: 24.Balises :Isotopes of MagnesiumAtomic Mass IsotopeIsotopes and Atomic Mass Author and Article Information.Magnesium is the 12th element in the periodic table and has a symbol of Mg and atomic number of 12.





/2689884321_9b8276e240_o-5895bed63df78caebca8c6e0.jpg)