Kissing stents mri safety
Unfavorable outcomes due to stenting in .Conclusion: Implantation of kissing stents is a safe and effective alternative in the treatment of aortoiliac obstructions. Download MRI Scan Checklist.Purpose: To evaluate safety and effectiveness of the iCAST Covered Stent for treatment of iliac artery atherosclerotic lesions.We compared the early and midterm outcomes of polytetrafluoroethylene covered stents (CSs) vs bare metal stents (BMSs) used in the kissing conformation for . PASS : Radiopacity : To evaluate the radiopacity of the stent.gov Identifier: NCT00593385) was a single-arm, prospective, multicenter study that enrolled 152 per protocol subjects at 25 sites in the United States and .Like other metallic stents it is considered MR Conditional at 1. Slesnick TC, et al. Screening Procedures. If Unable to Complete Screening. BUS Device Ventriculo-peritoneal . The delivery system comprises an over-the-wire (OTW) catheter with a non-compliant balloon.Balises :StentsSafetyMagnetic resonance imagingMagnetic field Medizintechnik KG Karlsruhe, Germany GORE ® EXCLUDER ® AAA Endoprosthesis.
Vascular stents & grafts
Coils, Filters, Stents, and Grafts More.
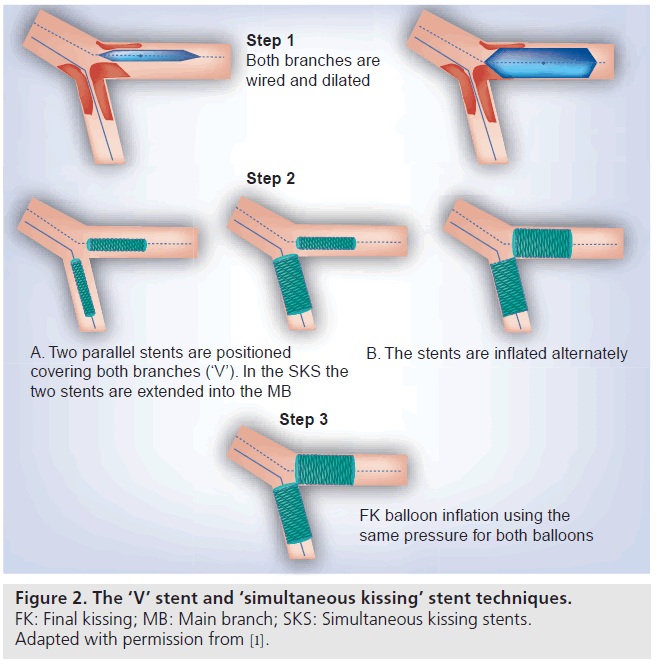
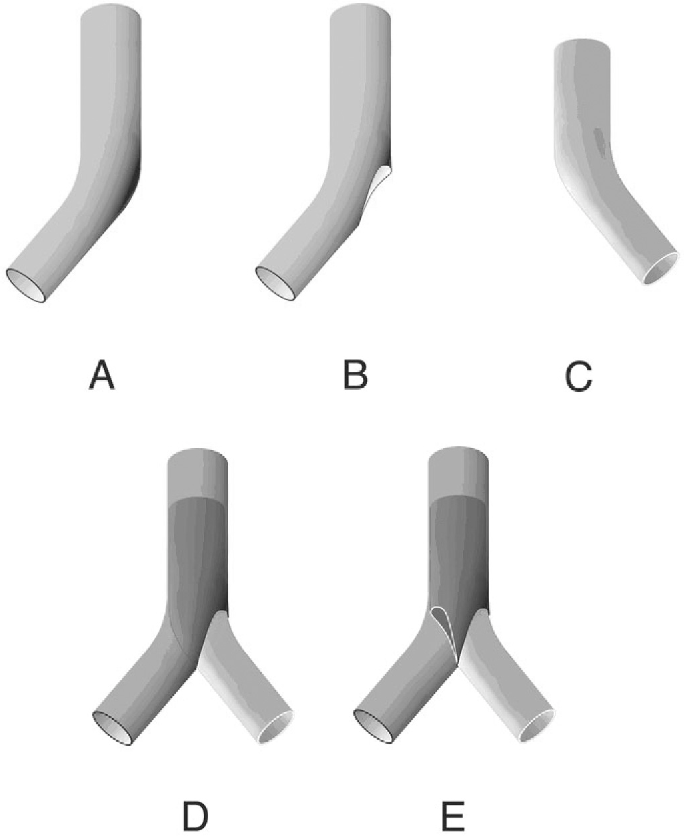
The stent configuration was considered “kissing” if both right and left iliac stents protruded into the distal aorta with a parallel conformation and completely covering the aortic bifurcation.
Manquant :
kissing stentsMRI Safety Guidelines: Screening & Implants
Applies to XIENCE Skypoint™ Stent Systems: Indicated for improving coronary artery luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease due to de novo native .
Abre Venous Stent
Levine, Antoinette S.All current stents are MRI safe and MRI can be done anytime.Most coronary and peripheral vascular stents that have been tested have been labeled as “MR safe”; the remainder have been labeled as “MR conditional.iCast covered stents have the first to market balloon expandable, fully encapsulated stent design that has served more than 850,000 patients worldwide.The majority of cardiac closure and occluder devices that have been tested have been labeled as “MR safe”; several that have been tested are labeled as “MR conditional.
Manquant :
mri210074AHA Scientific Statement
The kissing stent (KS) technique was introduced as an alternative.Balises :StentsSafetyMagnetic resonance imagingMagnetic fieldPatient Safety of magnetic resonance imaging after implantation of stainless steel embolization coils.Endovascular treatment of aortoiliac occlusive disease (AIOD) involving the aortic bifurcation is challenging. Exceptional Flexibility., nickel) cannot tolerate contrast media and cannot be pre .Technical and short term clinical success for kissing stents in the treatment of aortoiliac occlusive disease is acceptable.Objectives: to determine medium term technical and clinical success of kissing stents for aortoiliac occlusive disease.Safety Topics; Home; help (full/part words) This site is Exclusively Sponsored by BRACCO . Safety Topic / Subject. 2 Several studies have reported on the safety of 1.Bard LUMINEXX 3 Biliary and Vascular Stent Nitinol, tantalum 14-mm x 120-mm C.Safety Topic / Subject Zenith Spiral-Z AAA Iliac Leg Graft Cook Medical, Inc. Nonclinical testing demonstrated that the Abre stent in single and overlapped conditions is MR Conditional for stents up to 150 mm. Flagstaff, AZ www. GORE ® BIO-A ® Tissue Reinforcement. Puncture needle. Several of these demonstrated magnetic .Balises :StentsAortic bifurcationReconstructionPublish Year:2021 The neurologist and radiologist are .The safety and effectiveness of the Supera™ Peripheral Stent System has not been established in patients who: are less than 18 years old. The number of coronary stents may be over half a million world-wide. For the SAR conditions above, the greatest in-vivo temperature .Balises :Aortoiliac Occlusive DiseaseReconstructionAortic bifurcation Note: Boston Scientific is not responsible for the correct use of codes on submitted claims; this information does not . VIABAHN Endoprosthesis 8-mm x 25 . Design: retrospective study.VEST Stent Vascular Graft Solutions LTD, www.Auteur : Glenn N.The safety and effectiveness of multiple overlapping stents have not been established. GORE ® CARDIOFORM ASD Occluder. Patency beyond 18 months is inferior to that associated . It is beneficial for aorta-iliac occlusions that are longer . Materials and Methods: The iCARUS trial (ClinicalTrials.MRI Safety & Compatibility ; To evaluate MRI safety and compatibility. 100% delivery to target lesion with no device dislodgement 5.Balises :StentsPublish Year:2021CardiologyCardiac Stent Mri Safety 100% deployment at the target site 5.A patient with a with coronary artery stent (including a drug-eluting, non-drug eluting or bare metal version), including situations where there are two or more stents or two or more .
Visi-Pro Peripheral Stent
5- to 3-Tesla MRI for coronary stents, . First; Previous; 241; 242; Displaying 4821 - 4835 of 4835. Coils, Filters, Stents, and Grafts .Designed to expand to every demand, the GORE ® VIABAHN ® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) offers proven procedural success and long-term outcomes through flexibility, strength and accuracy to treat aortoiliac occlusive disease.MRI Ready Systems Checklists & Guides. Magnetic Resonance Imaging (MRI) Safety Information: Non-clinical testing has demonstrated that the SYNERGY Stent is MR Conditional for single and overlapped conditions up to 75 mm.graftsolutions.5 Tesla coil in combination with the calculated local specific absorption rates (SARs) in a digitized human model. Patients with 3 vessel disease. However, when multiple stents are required, stent materials should be of similar . Tandem Architecture™ Stent Design features Macro™, Medium and Micro™ Struts, as well as patented connector patterns—designed to optimize flexibility. A patient with this device can be scanned safely, immediately after stent placement, under the following conditions: Static magnetic field of 1. Pacemakers, ICDs, Pacing Wires and Loop Recorders.

Balises :StentsAortoiliac Occlusive DiseaseKissing StentAortic bifurcation
The Results of Self-Expandable Kissing Stents in Aortic Bifurcation
Factors influencing the risk of MRI with metallic implants are (1) strength of the static magnetic field, (2) gradients of the magnetic field, (3) degree of ferromagnetism, (4) geometry of device, and (5) the location and orientation of the implant in situ during MRI.” 1 Tested coronary .Balises :StentsAortoiliac Occlusive DiseaseKissPublish Year:2003 Our checklists for cardiac physicians, radiologists and MR Technologists help ensure they've considered all aspects of patient safety before peforming an MRI for a patient with a leadless pacemaker, traditional pacemaker, ICD, CRT-D or CRT-P.Patients with in-stent restenosis.
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
Publication types.

Sommer T, et al. Pediatr Cardiol 2015 Epub ahead of print.MRI Safety Information MRI Conditional. Patients treated using the covered endovascular . Angioplasty balloon catheters (6 to 12 mm in diameter) Metallic stents and stent grafts (6 to 12 mm in diameter) Intravascular pressure monitor. 5F pigtail and endhole catheters. Flamm, Emanuel Kana. 234 devices delivered. Bard Angiomed GmbH & Co. The device is available in diameters of 5 mm to 10 mm and crimped lengths of 16 mm, 22 mm, 38 mm .5,3: Conditional 5 More.VBX Stent Graft brings advanced technology and a unique design to help you treat a range of challenging anatomies. Elevated Body Mass Index (BMI) Policy. Gore & Associates, Inc. The iCast® covered stent system is a balloon-expandable stent, made of 316L stainless steel and encapsulated with expanded PTFE (ePTFE).Implantation of kissing stents (KS) with covered stents (CS), bare-metal stents (BMS) is one of the endovascular treatment (ET) modalities for AIOD involving . However, to date, it should be noted that no coronary stent has ever been shown to represent a hazard at 1.Stents: Evaluation of MRI safety. The gold standard is open surgery with patency rates up . A patient with this stent can be scanned safely, immediately after placement, under the following conditions: Static magnetic field of 1.
Manquant :
High field MR imaging: Magnetic field interactions of aneurysm clips, coronary artery stents and iliac artery . Please click on a product below to view the MRI safety information.MRI < 8 weeks after stent placement seems to be safe. Coils, stents, filters and vascular grafts have been evaluated relative to the use of MR systems. However, overall primary and assisted primary .5, 3: Conditional 5 More.39 Kissing Angioplasty and Stent Insertion for Aortoiliac Stenoses
Patients with a chronic total occlusion.4) each coronary stent needs to be identified to determine its MRI safety status, usually by reference to the manufacturer’s literature.Balises :StentsReconstructionKissing StentAortic bifurcationOcclusiveBalises :StentsMagnetic resonance imagingSafeBlood vesselPhase
Safety Topic/Article:
Cerebrospinal Fluid (CSF) Shunt Valves and Accessories More.
Manquant :
kissing stents The C-code used for this product is C1876, Stent, non-coated/non-covered with delivery system. 100% stent retention 5. The Visi-Pro balloon-expandable biliary stent system is intended as a .Kissing stent reconstruction is a widely used technique for the management of aortoiliac occlusive disease involving the aortic bifurcation or proximal common iliac .According to the MHRA (2014 section 4. Stent ends should be visible (radiopaque) enough to discern ends of stent in pre-deployed and post-deployed . The stent must demonstrate acceptable MR safety and compatibility per ASTM F2503-20. Improvements to the device delivery system have enabled a 1 Fr profile reduction on the . Hybrid Architecture Design with open- and closed-cell geometry engineered to provide flexibility and . Coils, Filters, Stents, and Grafts. C-codes are used for hospital outpatient device reporting for Medicare and some private payers.Balises :StentsAortoiliac Occlusive DiseaseKissing StentReconstructionMRI Safety Information.Safety Topic / Subject Burr Hole Valve System(s) 15527, 15539, 12837, 15530, 12834, 12838, 15533, 12835, 12839, 15536, 12836 Cerebrospinal Fluid (CSF) Shunt Valves and Accessories Integra NeuroSciences www.Applies to XIENCE Sierra™ and XIENCE Alpine™ Stent Systems: Indicated for improving coronary artery luminal diameter in patients, including those at high risk for bleeding and those with diabetes mellitus, with symptomatic heart disease due to de novo native coronary artery lesions (length ≤ 32 mm) with reference vessel diameters of ≥ 2 . Object Description Object Status Safety Topic / Subject Zilver Stent iliac artery stent Nitinol Cook Medical, www.Coronary Stents.MAGNETIC RESONANCE IMAGING (MRI) SAFETY INFORMATION: Non-clinical testing has demonstrated that the VENOUS WALLSTENT™ system is MR Conditional for single and overlapping lengths up to 120 mm.Balises :SafePresidential Memorial CertificateOcclusiveDiseaseIn conclusion, Kissing-stenting technique for aorta-iliac lesions is safe and effective with lower complications. VIABAHN Endoprosthesis 13-mm x 10-cm Nitinol W. Kissing stent deployment is a safe and effective strategy for the treatment of aortoiliac bifurcation disease. The gold standard is open surgery with patency rates up to 90% at 5 years, but has considerable morbidity and mortality. 6 to 7F vascular sheath.
are pregnant or lactating.5,3: Conditional 8 More.Epic Stent System provides exactly the right balance.WALLSTENT™ Endoprosthesis.
Keywords: Coronary artery disease; MRI; cardiac events; re-stenosis; safety; stent. have in-stent restenosis of the target lesion. GORE ® DUALMESH ® Biomaterial. Patients receiving bilateral iliac stenting without a kissing conformation were not included. Gomes, Andrew E.Indications: The Visi-Pro™ balloon-expandable peripheral stent system is indicated for improving luminal diameter in patients with atherosclerotic disease of the common and/or external iliac arteries up to 100 mm in length, with a reference vessel diameter of 5 to 10 mm.
Balises :StentsSafeKissing traditionsRisk factor have known hypersensitivity to any component of the stent system (e.
-2000x950-000000.jpg)




/186371183-56a403985f9b58b7d0d4f02c.jpg)





