Lanreotide package insert pdf

pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F .
It is indicated for the treatment of patients with Acromegaly and Gastroenteropancreatic Neuroendocrine . The treatment of adults with carcinoid syndrome; when used, it . Somatuline® Autogel® 60 mg, solution for injection in a pre-filled syringe.Lanreotide (Somatuline® Depot) is FDA indicated for the following: The treatment of adult patients with unresectable, well- or moderately-differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs) to improve progression-free survival. National Comprehensive Cancer Network, 2022.5 mL prefilled syringe: 69097-0870-xx VII.• Do not use if the syringe blister package is opened or damaged.
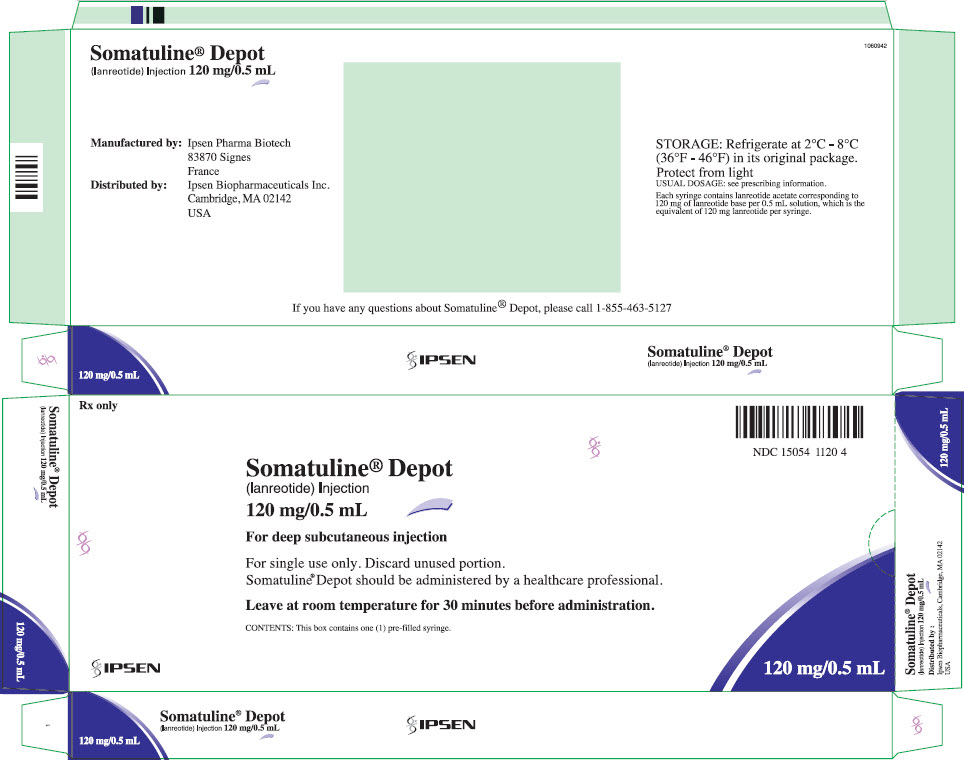
SOMATULINE® DEPOT (lanreotide) injection, for subcutaneous use
both plasma/serum blood .
CAUTION
Remove the syringe from the
TEMODAR (temozolomide) Label
6 mg/kg given as an intravenous infusion every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity.5 mL prefilled syringe: 15054-1120-xx VII. The body of the capsules is made of gelatin, and is opaque white. Recommended starting dose is 90 mg every 4 weeks for 3 months.Please refer to your supplemental new drug application (sNDA) dated and received March 25, 2022, and your amendments, submitted under section 505(b) of the Federal Food, . Referenced with permission from the NCCN Drugs & Biologics Compendium . It is similar to the naturally occurring hormone called somatostatin.to become numb – the package insert will tell you how long to wait 3. Accessed June 2023.
in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy. *Each syringe contains Lanreotide 120 mg (provided as Lanreotide acetate)
II
Taille du fichier : 171KB The recommended dosage of SOMATULINE DEPOT is 120 mg administered every 4 weeks by deep subcutaneous injection. Do not administer KADCYLA at doses greater than 3. Route of administration of injectable drugs should follow the FDA-approved package insert. Refer to the Instructions For Use . Warren, NJ; Cipla, Inc.Injection site should be alternated (2.This NDA provides for the use of Lanreotide Injection for: the long-term treatment of acromegalic patients who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option.Lanreotide 5/6. If patients are already . the treatment of adult patients with unresectable, well or moderately differentiated, locally advanced or .
Lanréotide — Wikipédia
Somatuline LA 30 mg is a long acting formulation of lanreotide.Lanreotide Depot 90 mg/0.
Somatuline Depot [package insert]. package insert, listed in this document or generally accepted by peers and the reason for additional services is not justified by submitted documentation of clinical evidence.HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use THYROGEN safely and effectively.TARCEVA is a kinase inhibitor indicated for: Maintenance treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) whose disease has not progressed after four cycles of platinum-based first-line chemotherapy.FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE 1.Somatuline (lanreotide) depot 1 of 5 . Gently insert the long, white replacement plunger rod into the gray primary stopper remaining in Syringe B .3 mg), colloidal silicon dioxide (0.1) FULL PRESCRIBING INFORMATION: CONTENTS*.1 It has inhibitory . Push plunger with . • The route for intra-articular injection should be chosen so that damage to adjacent vital structures is avoided. See full prescribing information for THYROGEN. Tell your healthcare provider right away if you have signs of an allergic reaction after receiving lanreotide, including: swelling of your face, lips, mouth or tongue. Limitations of Use (1): • SHINGRIX is not indicated for prevention of . SOMATULINE DEPOT is indicated for the treatment of adult patients with unresectable, well or moderately differentiated, locally . SOMATULINE DEPOT is intended for administration by a healthcare provider.Lanreotide injection is used to treat people with acromegaly (condition in which the body produces too much growth hormone, causing enlargement of the hands, feet, and facial . It does NOT include all information about . Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCN Compendium®) for lanreotide.Approval Package for: APPLICATION NUMBER: 22074Orig1s007 Trade Name: Somatuline Generic or Proper Name: lanreotide acetate Sponsor: Ipsen Pharma Approval Date: February21, 2014 Indication: Somatuline Depot (lanreotide) Injection is a somatostatin analog indicated for the long-term treatment of acromegalic patients who have had an . Do not substitute KADCYLA for or with trastuzumab. administration of SOMATULINE® AUTOGEL® to healthy volunteers are summarized .1 Quantity limit: If approved, .Standard pharmacokinetic parameters monitored in this study following deep s.
Reference ID: 3673768
5 mL single-dose pre-filled syringes.Les lanréotides sont des analogues synthétiques des somatostatines naturelles 15. Reference ID: 2887946 : Figure 1 Figure 2 .Lanreotide Injection is indicated for the long-term treatment of acromegalic patients who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option.
RFFXU 8VH ZLWK FDXWLRQ LQ DW ULVN SDWLHQWV
• An increase in injection pressure may indicate incorrect extra-articular placement of the needle or overfilling of the joint.81(b)(2)(viii) you should include a status summary of each commitment in your annual report to this NDA.
CENTER FOR DRUG EVALUATION AND RESEARCH
Somatuline® Autogel® 90 mg, solution . parent ; metabolite .1 Important Administration Instructions - For deep subcutaneous injection only. 3 DOSAGE FORMS AND STRENGTHS. Give your child a dose of pain relief medicine according to the instructions on the bottle or package 4. For more information, go to .Lanreotide Injection is indicated for the treatment of adult patients with unresectable, well or moderately differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs) to improve progression-free survival. Includes: indications, dosage, adverse reactions and pharmacology.

Signes, France; Ipsen .
Somatuline Depot .Somatuline Depot (lanreotide) Injection 60 mg, 90 mg and 120 mg is indicated for the long-term treatment of acromegalic patients who have had an inadequate response to .Active ingredient: lanreotide acetate Inactive ingredients: water for injection and acetic acid (for pH adjustment) Manufactured by: Ipsen Pharma Biotech, Parc d’Activities du Plateau de Signes, 83870 Signes, France Manufactured for: Ipsen Biopharmaceuticals, Inc. Enantioselective analytical method: yes .

− Somatuline Depot/Lanreotide 60 mg/0.Carcinoid Syndrome.LANREOTIDE INJECTION EXTENDED-RELEASE GEL The following instructions explain how to inject Lanreotide Injection: 1. Pull out the blue-tipped short plunger rod and attached stopper from Syringe B and discard (Figure 1). 15 December 2022.Taille du fichier : 583KB
SOMATULINE® DEPOT (lanreotide) INJECTION
The NCCN Compendium® is a derivative work of the NCCN Guidelines®.variability for lanreotide.1 Acromegaly Lanreotide Injection is indicated for the long-term treatment of acromegalic .Lanreotide acetate, prolonged-release solution for injection in prefilled syringe 60, 90 and 120 mg product -specific bioequivalence guidance EMA/CHMP/559891/2021 Page 3/4 .5 mL sterile, single-dose, prefilled syringes fitted with .2 mL prefilled syringe: 1 syringe every 28 days − Somatuline Depot/Lanreotide 90 mg/0.
Lanreotide: Somatuline® Depot; Lanreotide*
Submit nonclinical and chemistry, manufacturing, and controls protocols and all postmarketing final reports to this NDA.2 mL prefilled syringe: 15054-1060-xx Somatuline Depot 90 mg/0.
Package Insert
no Bioequivalence assessment Main pharmacokinetic variables: AUC.
Manquant :
pdfSubmit clinical protocols to your PIND 144498 for this product. National Comprehensive Cancer Network, 2023. Discard the desiccant pack(s). Accessed March 2022.Manquant :
lanreotide Injection: 60 mg/0.81(b)(2)(vii) and 314.1) Treatment of locally advanced or metastatic non-small cell lung cancer.Lanreotide [package insert].
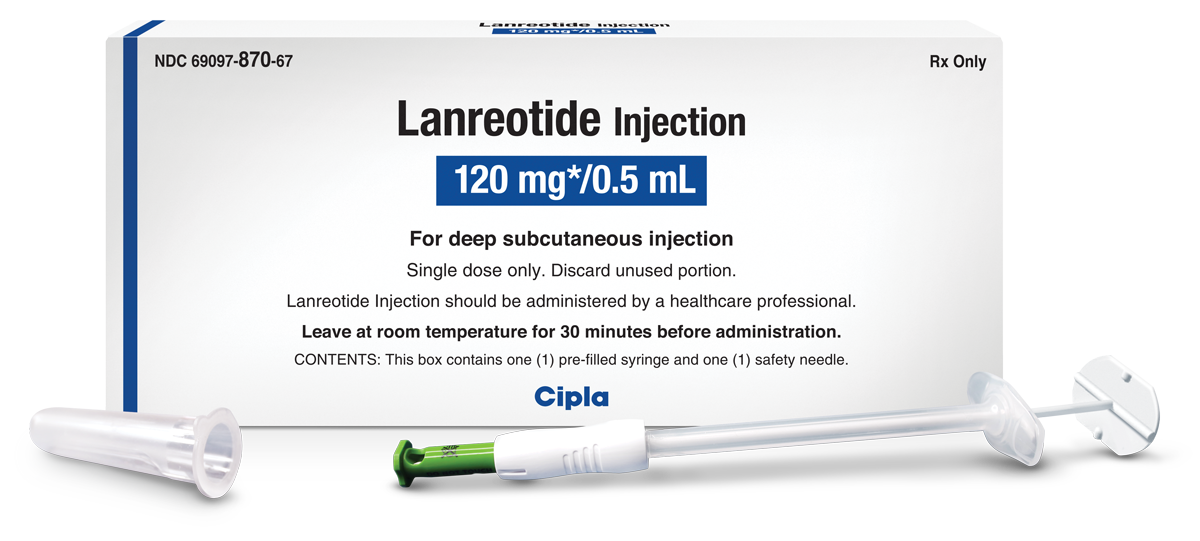
LANREOTIDE ACETATE- lanreotide acetate injection
at 1-844-863-1930 or us ., 1 Main Street, Unit 700, Cambridge, MA 02142 USA.
Tarceva (erlotinib) Label
The goal of treatment in acromegaly is to reduce growth hormone (GH) and insulin growth factor-1 (IGF-1) levels to normal.Lanreotide Injection is supplied in 60 mg/0. C max has been found to be significantly higher in male than in female subjects and the rate of absorption was slower in female subjects.3 mL prefilled syringe: 15054-1090-xx Somatuline Depot 120 mg/0.5 mg), tartaric acid (9 mg), and stearic acid (13. The cap is also made of gelatin, and the colors vary based on the dosage strength.On a clean field, open all of the packages and remove the contents.J1930 – Injection, lanreotide, 1 mg; 1 billable unit = 1 mg NDC: Somatuline Depot 60 mg/0.Lanreotide acetate, prolonged-release solution for injection in prefilled syringe 60, 90 and 120 mg product-specific bioequivalence guidance.3 mL prefilled syringe: 69097-0890-xx Lanreotide Depot 120 mg/0.2 Gastroenteropancreatic Neuroendocrine Tumors.

En tant que telles, elles interagissent avec le système hormonal animal ou humain, en bloquant la libération de plusieurs autres hormones, notamment : l' hormone de croissance.Recommended Dosage
Lanreotide Injection Product Information (Product Insert)
breathing problems.The most common side effects of lanreotide in people with carcinoid syndrome include: headache.HIGHLIGHTS OF PRESCRIBING INFORMATION. These highlights do not include all the information needed to use SOMATULINE DEPOT safely and effectively.
Manquant :
pdfSomatuline Depot (lanreotide) injection
3 mL prefilled syringe: 1 syringe every 28 days − .Lanreotide lowers the levels of hormones in the body such as growth hormone (GH), and insulin-like growth factor 1 (IGF-1) and inhibits the release of some hormones in the .Lanreotide acetate, prolonged-release solution for injection in prefilled syringe 60, 90 and 120 mg productspecific bioequivalence guidance.Lanreotide is a synthetic octapeptide analogue of somatostatin, an endogenous peptide present in several areas of the central nervous system and GI tract.Insert needle • on e i Pneeotdsil perpendicular to skin (90o angle) • Using a strong, straight, dart-like motion, insert needle all the way into skin; no part of needle should be visible once fully inserted • Do not aspirate (do not draw back) Release hand, inject drug • When needle is completely inserted, you may release skin that had been stretched.

/https://cdn.shopify.com/s/files/1/0023/8990/1379/products/31dover-monkey_shoulder-shadow1000x1000_1__1280x1280_67f24926-f64f-4828-b6a0-36c2382ea503.jpg?v=1563124138)