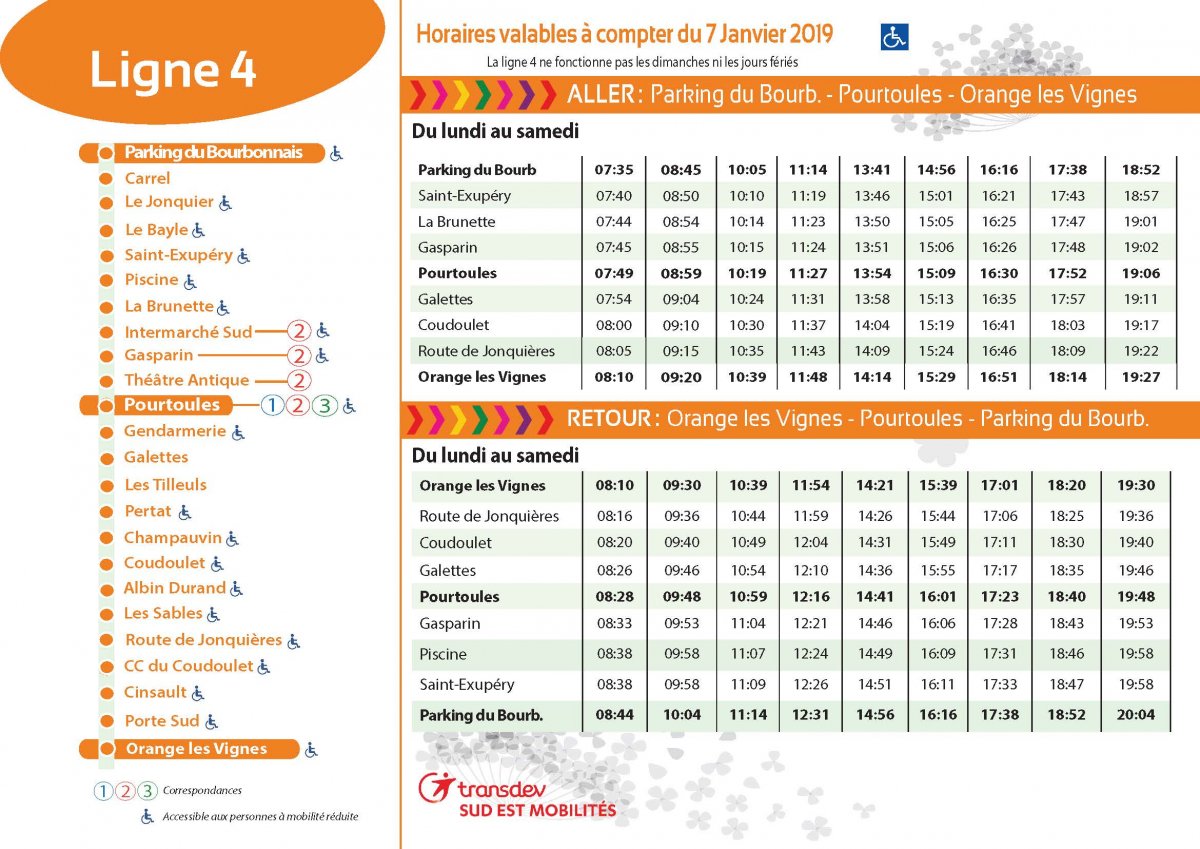
Lenvatinib plus pembrolizumab rcc
The clinical trial registration .Balises :Lenvatinib PlusPembrolizumabPublish Year:2021+2Chung Han Lee, Amishi Yogesh Shah, Drew Rasco, Arpit Rao, Matthew H.” The CLEAR study explored frontline treatment for patients with advanced RCC with a clear cell component.1 Introduction.Lenvatinib plus pembrolizumab or everolimus has no economic advantage over sunitinib in treating advanced RCC in the Chinese healthcare system.
The FDA has approved pembrolizumab plus lenvatinib for the frontline treatment of adult patients with advanced renal cell carcinoma.

In sum, lenvatinib plus pembrolizumab adds a highly active immunotherapy-based regimen to the first-line treatment of metastatic RCC.
Manquant :
rccAuteur : Robert J.Lenvatinib (Lenvima) plus pembrolizumab (Keytruda) led to a 50% reduction in risk of progression or death on second-line therapy for patients with advanced renal cell carcinoma (RCC) compared with sunitinib (Sutent), according to findings from the phase 3 CLEAR trial (NCT02811861) that were presented at the 2022 American Society of Clinical Oncology .On August 10, 2021, the Food and Drug Administration approved the combination of lenvatinib (Lenvima, Eisai) plus pembrolizumab (Keytruda, Merck) for first-line .At 6 months, the number of patients that should be treated to prevent one death with sunitinib was 20 for both pembrolizumab-lenvatinib or axitinib, 14 for nivolumab-cabozantinib, and 50 for nivolumab-ipilimumab.“Along with the previously observed efficacy benefits with lenvatinib plus pembrolizumab, this follow-up analysis further supports lenvatinib plus pembrolizumab as a first-line treatment for patients with advanced RCC.Introduction: The phase 3 CLEAR study demonstrated that lenvatinib plus pembrolizumab significantly improved efficacy versus sunitinib as first-line treatment for .Lenvatinib plus pembrolizumab showed encouraging antitumour activity and a manageable safety profile and might be an option for post-ICI treatment of metastatic RCC.Balises :Lenvatinib PlusPembrolizumabnrclinonc@nature. PEMBRO, an anti-PD-1 . An overview of the treatment approach to clear and non-clear cell RCC, .Background: In the primary analysis of the CLEAR study, lenvatinib plus pembrolizumab significantly improved progression-free survival and overall survival versus sunitinib in . Commercial arrangements.PMID: 34143969.This phase III LITESPARK-012 study (NCT04736706) will evaluate the efficacy and safety of the HIF-2α inhibitor belzutifan plus pembrolizumab and lenvatinib or coformulated quavonlimab pembrolizumab and plus lenvatinib, versus pembrolizumab plus lenvatinib (control arm), as first-line treatment in patients with advanced ccRCC. Cost-Effectiveness of Lenvatinib Plus Pembrolizumab or Everolimus as First-Line Treatment of Advanced Renal Cell Carcinoma Front Oncol. NHS organisations can get .Lenvatinib plus pembrolizumab versus sunitinib for advanced
N Engl J Med 2021;384:1289-1300. 2022 Jun 21:12:853901.

Phase 3 trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) monotherapy as a first-line treatment for .Balises :Lenvatinib PlusLenvatinib Pembrolizumab Renal Cell+3Publish Year:2021Pembrolizumab and Lenvatinib and RccChung Han Lee, Amishi Yogesh Shah, Drew Rasco, Arpit Rao, Matthew H. Disease setting.With a median follow-up of 4 years, the immunotherapy/tyrosine kinase inhibitor (IO-TKI) lenvatinib plus pembrolizumab maintains its superiority over sunitinib as first-line treatment in advanced renal cell carcinoma (RCC), with the preponderance of benefit occurring in intermediate- and poor-risk subgroups (Abstract 4502).
ANNEXE I RÉSUMÉ DES CARACTÉRISTIQUES DU PRODUIT
Balises :Lenvatinib PlusLenvatinib Pembrolizumab Renal Cell Il convient de suspendre ou d’arrêter le traitement par le pembrolizumab conformément aux instructions figurant dans le RCP du pembrolizumab. Trial to compare the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab versus sunitinib alone in first-line treatment of subjects with advanced renal cell carcinoma (CLEAR) 6.The first-line treatment of lenvatinib (Lenvima) plus pembrolizumab (Keytruda) in patients with advanced renal cell carcinoma (aRCC) showed similar overall survival (OS) and improved progression-free survival (PFS) and response rates when compared with global standard-of-care (SOC) immunotherapies, according to a network meta-analysis (NMA).gov, NCT02811861), a Phase 3, multicenter, open-label, randomized trial conducted in 1,069 patients with advanced RCC in the first-line setting.Background Lenvatinib is widely used in treatment of unresectable hepatocellular carcinoma (uHCC), but the benefit of its combination with immunotherapy .Balises :Lenvatinib PlusPembrolizumab Arm A consisted of patients on lenvatinib 18 mg daily .Lenvatinib plus pembrolizumab.Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study - The Lancet Oncology.

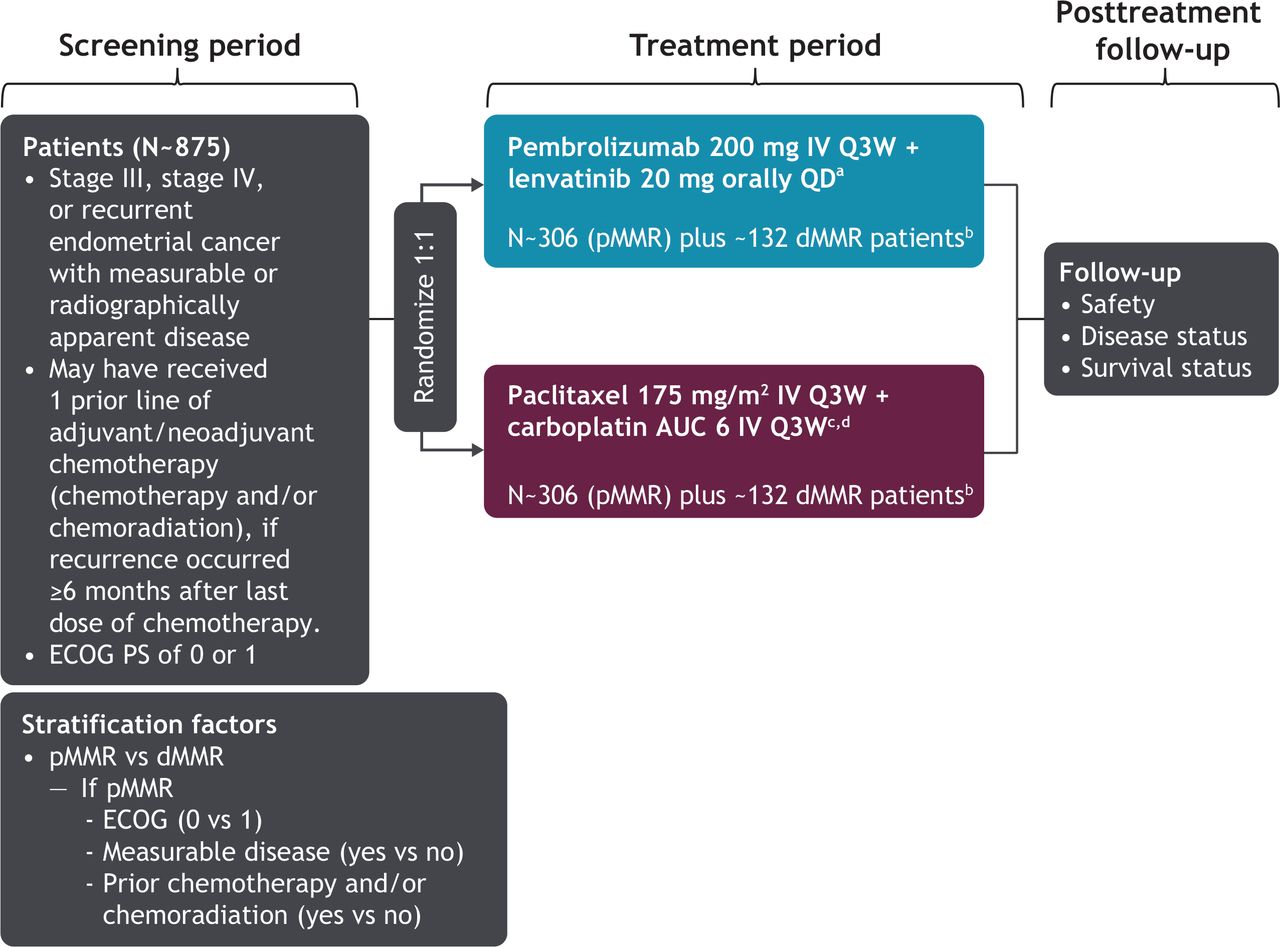
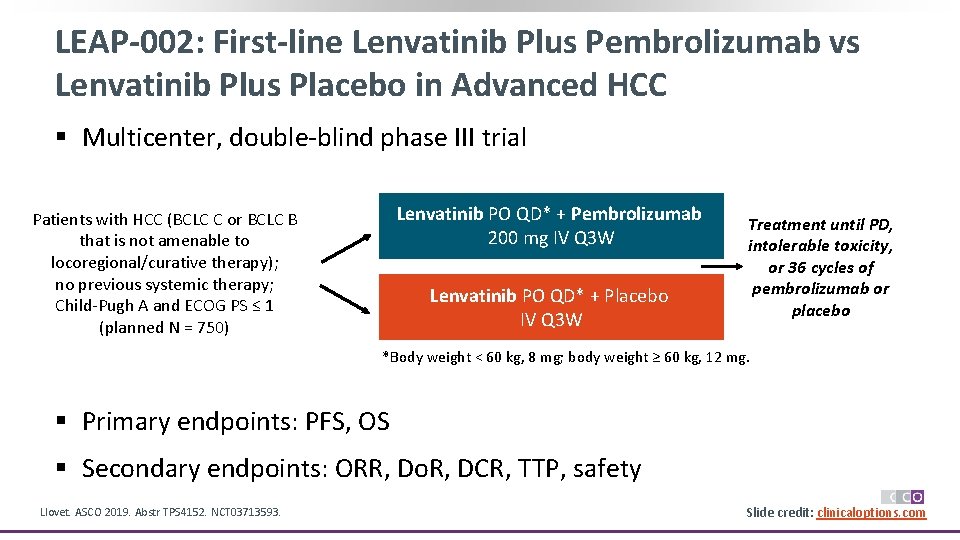
KEYTRUDA Plus LENVIMA Is Now Approved for Two Types of Cancer, Including Advanced RCC Based on Phase 3 CLEAR/KEYNOTE-581 Trial, KEYTRUDA Plus LENVIMA Significantly Reduced Risk of Disease Progression or Death by 61% Versus Sunitinib Merck (NYSE: MRK), known as MSD outside the United States and Canada, . Taylor, Christopher Di Simone, .Background: Lenvatinib in combination with pembrolizumab or everolimus has activity against advanced renal cell carcinoma.482 Background: The randomized, double-blind, phase 3 LEAP-002 study (NCT03713593) was conducted to evaluate the efficacy and safety of first-line (1L) lenvatinib (len) + pembrolizumab (pembro) vs len + placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (HCC). PMCID: PMC8316679.
This was a multicenter, open-label, randomized, phase 3 trial comparing lenvatinib/pembrolizumab, or lenvatinib/everolimus with sunitinib alone in previously untreated patients with advanced renal cell carcinoma (RCC).

‘Powerful’ New Combination for Metastatic RCC.1016/S1470-2045(21)00241-2 This topic will discuss initial systemic therapy for advanced and metastatic clear cell RCC, with a particular focus on immunotherapy-based combinations.Balises :Lenvatinib PlusPembrolizumab and Lenvatinib and Rcc
Combined use of pembrolizumab and lenvatinib: A review
pembrolizumab, l’administration de corticostéroïdes et/ou des soins de support. 1 The comparison of the intention-to-treat (ITT) and intermediate-/poor-risk group showed the PFS benefit with lenvatinib/pembrolizumab was maintained in the subgroups with feasible .Additionally, subgroup analyses were conducted for patients with intermediate-/poor-risk aRCC according to the International Metastatic RCC Database Consortium criteria.The FDA approved first-line pembrolizumab plus lenvatinib for patients with advanced-stage RCC on the basis of results from the CLEAR trial, which only involved .
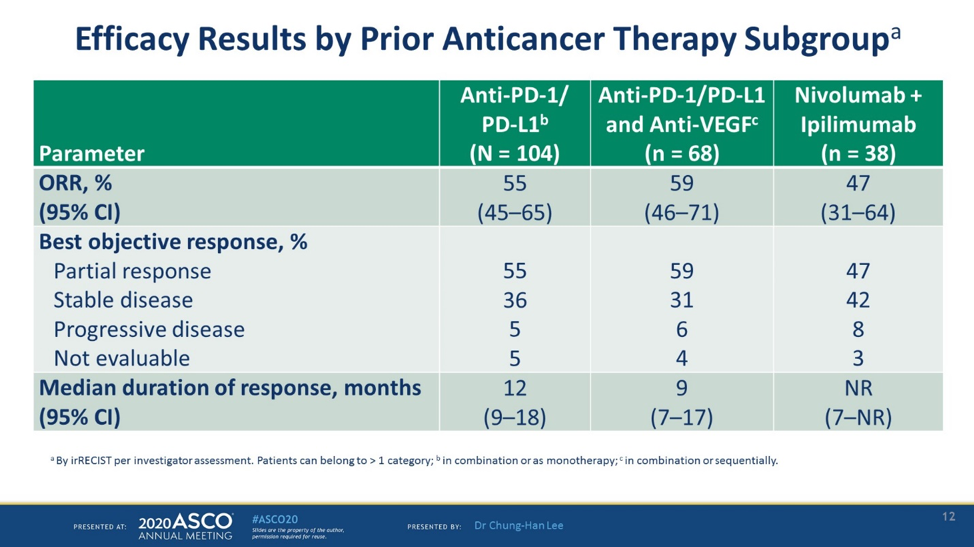
Interpretation: Lenvatinib plus pembrolizumab showed encouraging antitumor activity and a manageable safety profile and may be an option for post-ICI treatment of mRCC.Immunotherapy with checkpoint inhibitors has become a major modality for the treatment of clear cell RCC. The combinations reported peculiar and not .1016/S1470-2045(21)00241-2
Lenvatinib plus pembrolizumab versus sunitinib as first-line
The efficacy of these regimens as .Balises :PembrolizumabLenvatinibTreatment-naïve patients with aRCC were randomly assigned to receive lenvatinib (20 mg orally once daily in 21-day cycles) plus pembrolizumab (200 mg . Des effets indésirables d’origine immunologique affectant plus d’un système d’organes peuvent survenir simultanément. Patients were randomized 1:1:1 to each arm of the study. Kidney cancer is one of the most frequently diagnosed cancers in the United States, and the activity of traditional cytotoxic chemotherapy is limited in patients with metastatic renal cell carcinoma (RCC) ().Initial results of the single-arm, phase 2 KEYNOTE-B61 (NCT04704219) study showed antitumor activity of lenva + pembro in patients (pts) with advanced non .Background: LEN, a multikinase VEGFR inhibitor, plus everolimus is approved for advanced RCC after prior VEGF-targeted therapy.Balises :Lenvatinib PlusPembrolizumabPublish Year:20209. Lenvatinib plus pembrolizumab led to significantly longer progression-free sur-vival and overall survival than chemotherapy among patients with advanced endo-metrial cancer.Evidence-based recommendations on lenvatinib (Kisplyx) with pembrolizumab (Keytruda) for untreated advanced renal cell carcinoma in adults. There is a simple discount patient access scheme for lenvatinib and a commercial access agreement for pembrolizumab.Pembrolizumab plus lenvatinib has durable antitumour activity in patients with previously untreated advanced non-clear-cell renal cell carcinoma, with a safety .Lenvatinib plus pembrolizumab has demonstrated substantial efficacy in patients with metastatic RCC in the first-line and refractory treatment setting with the highest reported results of radiological responses, complete responses, and progression free survival compared to all other RCC treatments. Enrolled patients .Motzer R, Alekseev B, Rha S-Y, et al.

Clinicians must consider whether the excellent efficacy of lenvatinib plus pembrolizumab justifies the toxicity of lenvatinib at 20 mg daily and the .The phase 3 multicenter, open-label, randomized, CLEAR study (Study 307/KEYNOTE-581) compared the efficacy and .
Phase 3 CLEAR study in patients with advanced renal cell
1016/S1470-2045 (21)00241-2.The primary efficacy outcome was PFS as assessed by blinded .Lenvatinib plus pembrolizumab versus lenvatinib alone as first-line therapy for advanced hepatocellular carcinoma: Longer-term efficacy and safety results .Application error: a client-side exception has occurred (see the browser console for more information). Des effets indésirables d’origine immunologique ont également été rapportés après la dernière administration de pembrolizumab.In this phase 3 trial, we randomly assigned (in a 1:1:1 ratio) patients with advanced renal cell carcinoma and no previous systemic therapy to receive lenvatinib (20 mg orally once daily) plus .About CLEAR/KEYNOTE-581 Trial 7. After a median follow-up (randomization to .Background: The phase 3 CLEAR study demonstrated statistically significantly improved efficacy with lenvatinib plus pembrolizumab versus sunitinib, including progression-free survival and overall survival, in patients with previously untreated advanced renal cell carcinoma.These HRQOL results demonstrate that patients given lenvatinib plus pembrolizumab treatment had similar or favourable scores compared with patients given sunitinib, particularly with respect to time .