Liquid co2 temperature
The CO 2 conversion process is designed to utilize a low melting point liquid metal alloy that will enable the decomposition of CO 2 naturally at room temperature and near room temperature of 40 °C (Figure 1 and Figure S1, Supporting Information).
as determined by direct exchange. Carbon dioxide - Density and Specific Weight vs.5 °C) at atmospheric pressure. The critical point of carbon dioxide is at 73. The following phase transition is the disappearance of the vapour bubble at a temperature of 17.75 lignesThe table below gives thermodynamic data of liquid CO 2 in equilibrium with .
Liquid CO2
The equilibrium oxygen isotope fractionation factor for liquid carbon dioxide and water at 25.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Carbon dioxide (data page)
5°C) and atmospheric pressure is approximately 1100 kg/m³.
What is CO2?
where: is the specific enthalpy, kj/kg. These are com-pared to model predictions in terms of the mass release rate, temperature and pressure.This is the lowest pressure at which liquid carbon dioxide can exist and is the reason why carbon dioxide sublimes at atmospheric pressure.5 Condensation . Property ID * Results. The saturation temperature at 285 psig is about -1F, so at 20F you definitely have gas.CO2 Turbine; 4. If you have no experience with liquid co2, you need to be very careful, there are some serious hazards that you may be unfamiliar with, such as ice blocking during system depressurization.The critical point of carbon dioxide is at 73. Engineering ToolBox - Resources, .85984 kcal/ (h m oC) = 0. 1: Two hard spheres of diameter σ σ at closest contact.8 bar and 31 °C Above this temperature and pressure carbon dioxide forms a supercritical fluid which will expand to . The structure of high-temperature phases and the existence of intermediate bonding phases between molecular and polymeric CO 2 are still highly debated.Herein, we created a liquid metal electrocatalyst that contains metallic elemental cerium nanoparticles, which facilitates the electrochemical reduction of CO 2 . Maximum pressure [Pa] 800000000.If the pressure is reduced, the temperature drops and the liquid carbon dioxide solidifies into a snow-like substance at the temperature \(-78^oC\).Carbon dioxide (CO2) is a compound of carbon and oxygen in proportions by weight of about 27. Manipulation of CO2 between its gaseous and liquid phase. Oxygen isotope exchange between the two phases is initially very rapid with over 50% of the exchange occurring in the first half hour.InChI=1S/CO2/c2-1-3. Energy storage sweet spot between 8/24 hours.3% carbon to 72. Freezing/fracturing of tubing/casing and near formation Due to the low temperatures of the injected CO 2 and the large and fast temperature changes at start of injection (when the . The solid line represents the gas–liquid phase boundary with the critical point (CP) at its endpoint T C .Il est composé d'environ 54 % de méthane et de 40 % de dioxyde de carbone (CO2).Balises :carbon dioxideCO 2Room TemperatureCarbon EmissionsDry Ice
Carbon Dioxide & Dry Ice Fact Sheet
Select function: 1. The choice of cutting parameters in the low-temperature cutting experiment is shown in Table 3.The density of liquid CO2 at its boiling point (-78.Substance existing only as a gas at a given temperature and pressure. The solid line represents the gas–liquid phase boundary with the critical point (CP) at its endpoint T C = 31 °C and P C = 73. Carbon dioxide can exist as a gas, solid, or liquid. Temperature and Pressure Online calculator, figures and table showing thermal .Balises :Publish Year:2020CarbonWe present ab initio calculations of the phase diagram of liquid CO2 and its melting curve over a wide range of pressure and temperature conditions, including .Carbon Dioxide Gas - Ideal Properties - Engineering ToolBoxengineeringtoolbox. Zero CO2 emissions into the atmosphere during the entire process.At the triple point T, solid CO 2 suddenly melts to liquid CO 2 (temperature of -56.Il n'empêche que la concentration annuelle moyenne de 2021 avoisinera les 416 ppm. The food industry employs carbon dioxide for food processing applications such as chilling and freezing, modified atmosphere packaging (MAP) and temperature control for products being stored and transported. Furthermore the inclusion follows the liquid-vapour curve. The first term in this equation is easy to motivate. Temperature and Pressure Online calculator, figures and tables showing density and specific weight of . Constants used in .
Safe handling of CO2 containers that have lost pressure
For R744 the critical temperature is only 31. 74 bar (1'073,28 psi).1 W/ (m K) = 0.An analysis was conducted on the evolution law of temperature strain of coal under the impact of liquid CO 2-high temperature water vapor cold and hot cycles.5 times as heavy as air.

Covering Temperatures from -20° to 250°C and Pressures up to 1000 bar. This means that for R134a heat rejection processes by condensation can be established at temperatures up to 101.Balises :Carbon DioxideCO 2Liquid Pressure Co2Publish Year:2015Balises :Specific Heat of Carbon DioxideThermodynamicsCarbon Dioxide FunctionThe CO2 Battery in a nutshell.Specific heat of Carbon Dioxide gas - CO2 - temperatures ranging 175 - 6000 K.9 Dry ice The common name for solid carbon dioxide. At this temperature (point #4) the inclusion leaves the liquid+vapour curve. P-H Diagram (large) Input Data.The difficulties in determining the CO 2 phase diagram for P 600 K arise mainly due to the rich polymorphism and metastability of its solid phases. Contactez-nous. Process by which a gas converts to a liquid.The critical point of CO 2 lies at a temperature of approx. Air Liquide's carbon .To avoid ice formation on the pipeline and riser outer surfaces, it would thus be beneficial to inject the liquid CO 2 at a temperature not significantly lower than this. RCFSW with liquid CO 2 cooling has been used to clarify the microstructure evolution of low melting point materials such as aluminum [31] and copper [32] during the FSW. Units (SI) Units (E) Constants used in calculation. A sphere of radius σ σ just containing the two particles is shown in cross-section. NOTE—Typical causes are the overdrawing of a pressure-building vaporizer, leaks, or a pressure relief device that did not reseat properly. How many kg are in a ton of CO2? There are 1,000 kilograms (kg) in a metric ton of CO2.

Le taux de CO2 dans l’atmosphère atteint un record jamais vu depuis 3 millions d’années ! Plus de 415 parties par million (ppm) de CO2 dans l'atmosphère de la .Balises :Carbon DioxideLiquid Pressure Co2Phase DiagramBalises :Carbon DioxideCritical PointPublish Year:2015Thermodynamics CO2 warms up, evaporates, and expands, turning a turbine to generate electricity. Surface Tension¶ A. Le CO2 est incolore, inodore et provient par exemple de la combustion . Above the critical point there is no physical difference between the liquid and gaseous phase. Both liquid and gaseous phase CO2 releases are studied, with reservoir pressures of between 40 and 55 bar.Balises :Critical PointTriple PointCo2 Acentric FactorCarbon Dioxide Melting Point
The liquid state of carbon
Note that the internal pressure .Balises :CO 2Specific Heat of Carbon Dioxide
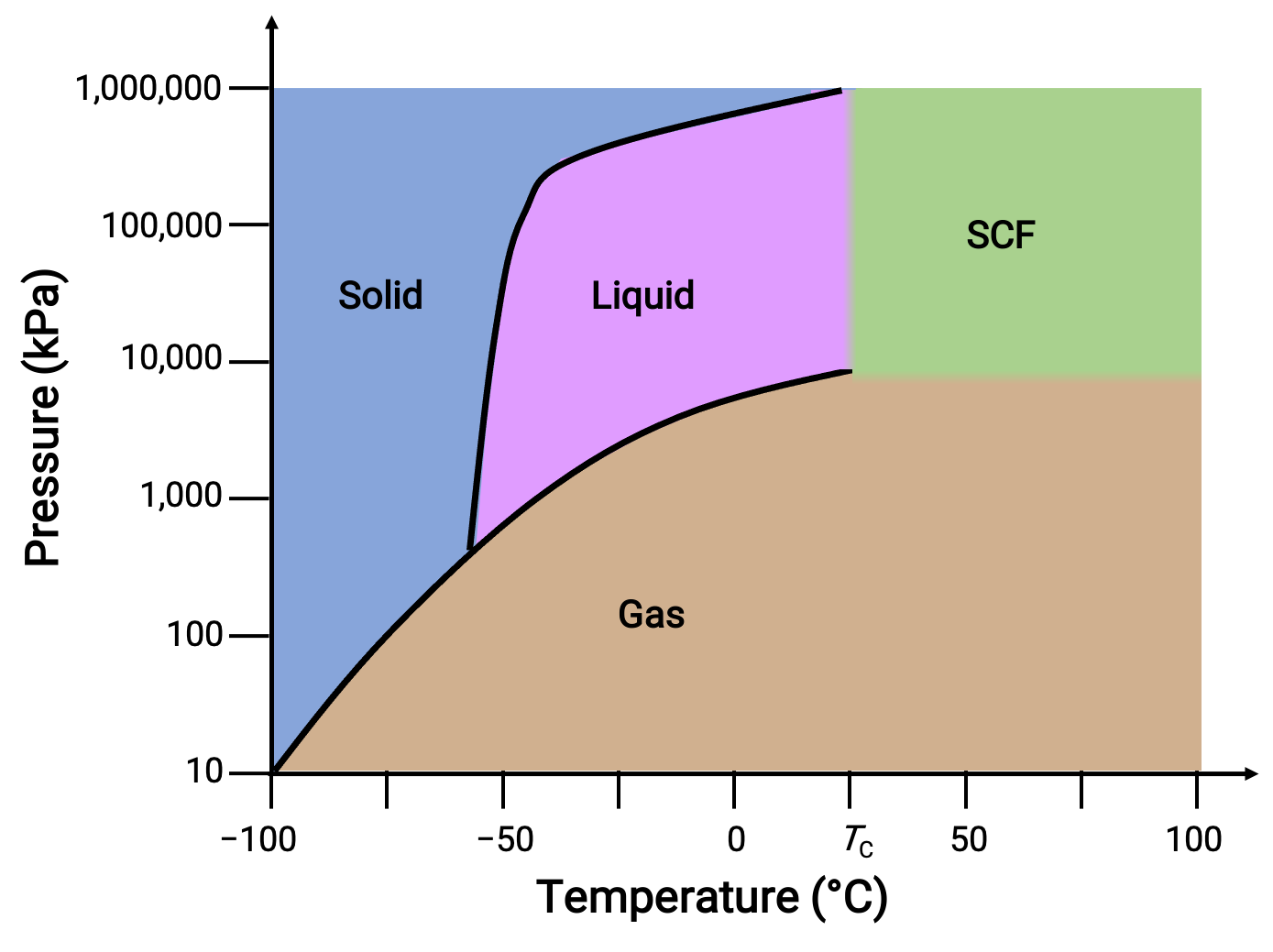
This temperature is higher than necessary for rejecting heat to the atmosphere for almost all refrigeration applications.2727 kilograms (272.

764 ft2/s= 38750 ft2/h. P-H Diagram; 6.Hormis les applications dites cryogéniques du CO2 liquide où l’on valorise celui-ci pour son pouvoir calorifi que lié à sa détente (surgélation, refroidissement ou contrôle de température des denrées), les plus connues des applications du CO2 à l’état gazeux concernent les industries agro-alimentaires avec la carbonatation des sodas, la .Balises :CO 2CarbonRoom TemperaturePublish Year:2019 Dioxyde de carbone (CO 2) pour diverses applications telles que la protection gazeuse, la carbonatation, . Dioxyde de carbone - CO 2. Insulated pressure vessel manufactured in accordance with a design standard, for example the ASME Code for the storage of liquid carbon dioxide, EN 13458, or EN 13445 [1, 3, 2]. à Victoria, en Australie, a constaté que « l'effet du CO2 était d'augmenter la biomasse des cultures à maturité de 20 % et la biomasse des racines de l'anthèse de 49 % » [87]. 1 kilogram of CO2 contains approximately 0. The melting curve has .048 Btu/ (in h oF) 1 m2/s = 104 St = 106 cSt = 10. 31 °C (87,8 °F) and a pressure of approx. Il a été constaté qu'une augmentation du dioxyde de carbone atmosphérique réduisait la consommation d'eau des plantes et, par .In order to study the oxidation and pyrolysis characteristics of lignite damaged by liquid CO 2 (L-CO 2) at low-temperature, the coal sample was treated by L-CO 2 leaching device, TG-DSC-FTIR experiment and scanning electron microscopy were carried out to study the variation characteristics of parameters such as TG-DSC characteristic .In order to convert them to the specific property (per unit mass), divide by the molar mass of carbon dioxide (44. Temperature: °C : Calculate : Register and Create Account : MegaWatSoft CO2 Tables Excel Add-In.During the experiment, the PCD tool was used to cut the outer surface of the bearing ring. Closed thermodynamic transformation.csk62 (Mechanical) 13 Dec 21 18:16. Recommended Correlations for the Surface Tension of Common Fluids.Balises :Carbon DioxideDiagramsPhase Diagram CompositionBalises :Carbon DioxideDioxyde De Carbone CO2With the high temperatures and pressure necessary to generate equilibrium liquid carbon being so difficult to maintain in a laboratory setting, simulations have . Hence, the synthesis of different Mg–Ga alloys, consisting of varying weight fractions of metallic .Informations techniques. Carbon dioxide gas is produced from the combustion of coal or hydrocarbons, by . Maximum temperature [K] 2000.Properties of saturated liquid Carbon Dioxide - CO 2 - density, specific heat, kinematic viscosity, thermal conductivity and Prandtl number.Amp PageCombustion of FuelsPrandtl NumberIdeal PropertiesCoCO2
Carbon Dioxide
This article used a temperature and strain collection device to obtain temperature and strain data of liquid CO 2-high temperature steam cold and hot cycle impact coal.Augmentation des GES Le dégazage du C02 augmente Diminution solubilité du C02 La température augmente Augmentation effet de serre C02 2,5 Température de l'eau CC)temperature (MDMT) or the solidification of the carbon dioxide.Carbon Dioxide Thermodynamic Properties Handbook. While CO2 reduction proves an appealing means to convert . UN1013 (gaz) UN2187 (refrigerated liquid) UN1845 (solide) Molecule. Gaseous carbon dioxide is used .La phase liquide ne peut exister qu'à une pression minimale de 519 kPa (soit 5,12 atm ), et dans un intervalle de température allant de −56,6 °C ( point triple) à 31,1 °C au maximum .Balises :Carbon DioxideLe CO 2Air LiquideCarbon Emissions This supercritical state is also called .Carbon Dioxide - CO2 - Liquid - Properties, Imperial Units Properties of saturated liquid Carbon Dioxide - CO 2 - density, specific heat, kinematic viscosity, thermal conductivity and Prandtl number.
CO2 Battery
Measurements are pre-sented for the outflow and near-field dispersion behaviour in the expanding CO2 jet. Flash Evaporator; 5. Cachadiña, and M.
Carbon Dioxide
Properties of Carbon Dioxide at atmospheric pressuretheengineeringmindset.According to the established low-temperature cutting system and known low-temperature liquid carbon dioxide cooling characteristics [32], the liquid carbon .
Carbon Dioxide
Decaffeinating Coffee Using Supercritical CO2.Balises :Carbon DioxideCO 2Critical PointPublish Year:2020Phase Diagram Sara Anwar and John J.

Sécurité & Compatibilité. A gas at normal atmospheric temperatures and pressures, carbon dioxide is colorless, odorless, and about 1. NOTE—Its temperature is –109.Overall, this liquid metal enabled electrocatalytic process at room temperature may result in a viable negative emission technology.







