List of metals

The following table provides a comprehensive list of density values for pure metals at room temperature (approximately 25°C or 77°F) and 1 . 6) Shear strength: the maximum shear load a material can withstand before failing divided by its cross-sectional area.
Metal Strength Chart
Ore - Ores are naturally occurring rocks that contain sufficient amounts of metals or metal compounds to make extraction feasible.0 g/cm3, aluminum is 2.Metals: lose electrons to form cations when reacting with non-metals. The metalloid group separates the metals from the nonmetals. This category has the following 20 . Certaines listes incluent le mercure comme métal noble .
List of Metals
Les métaux sont des éléments chimiques qui possèdent des propriétés physiques et chimiques uniques, leur permettant d’être utilisés dans une variété de domaines, . Find out the properties, occurrence, .
Table of Electrical Resistivity and Conductivity
Density of Metals – List of Metals by Density., the number of equidistant nearest neighbors) is . The lower the resistivity, the more readily the material permits the flow of electric charge. 9 out of 15 constituents of the Nifty Metal index have shown impressive returns, ranging between 20% and 50%, with Vedanta leading as the . Galvanic corrosion is of particular concern in design and material selection. Metalloids can also be called semimetals. Examples are calcium oxide, aluminum oxide, ferric oxide, potassium oxide, etc. The iron makes the metal prone to corrosion and imparts magnetic properties. Element 31 - Gallium.

List of chemical elements
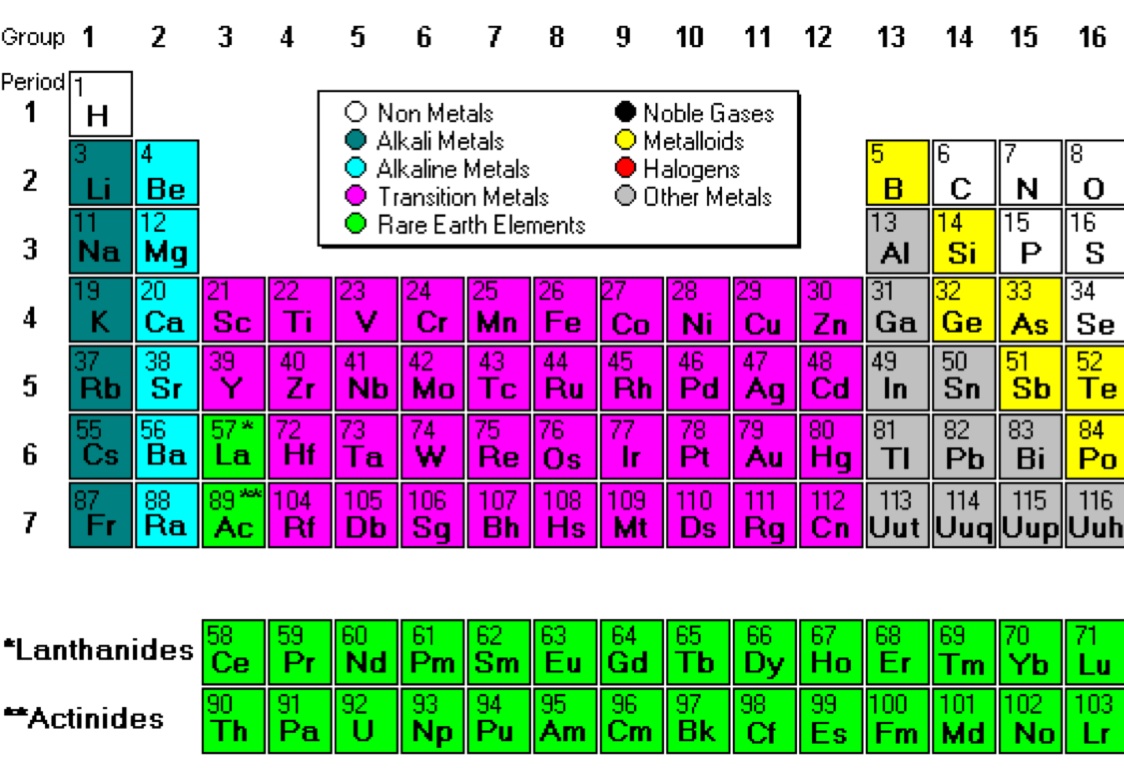
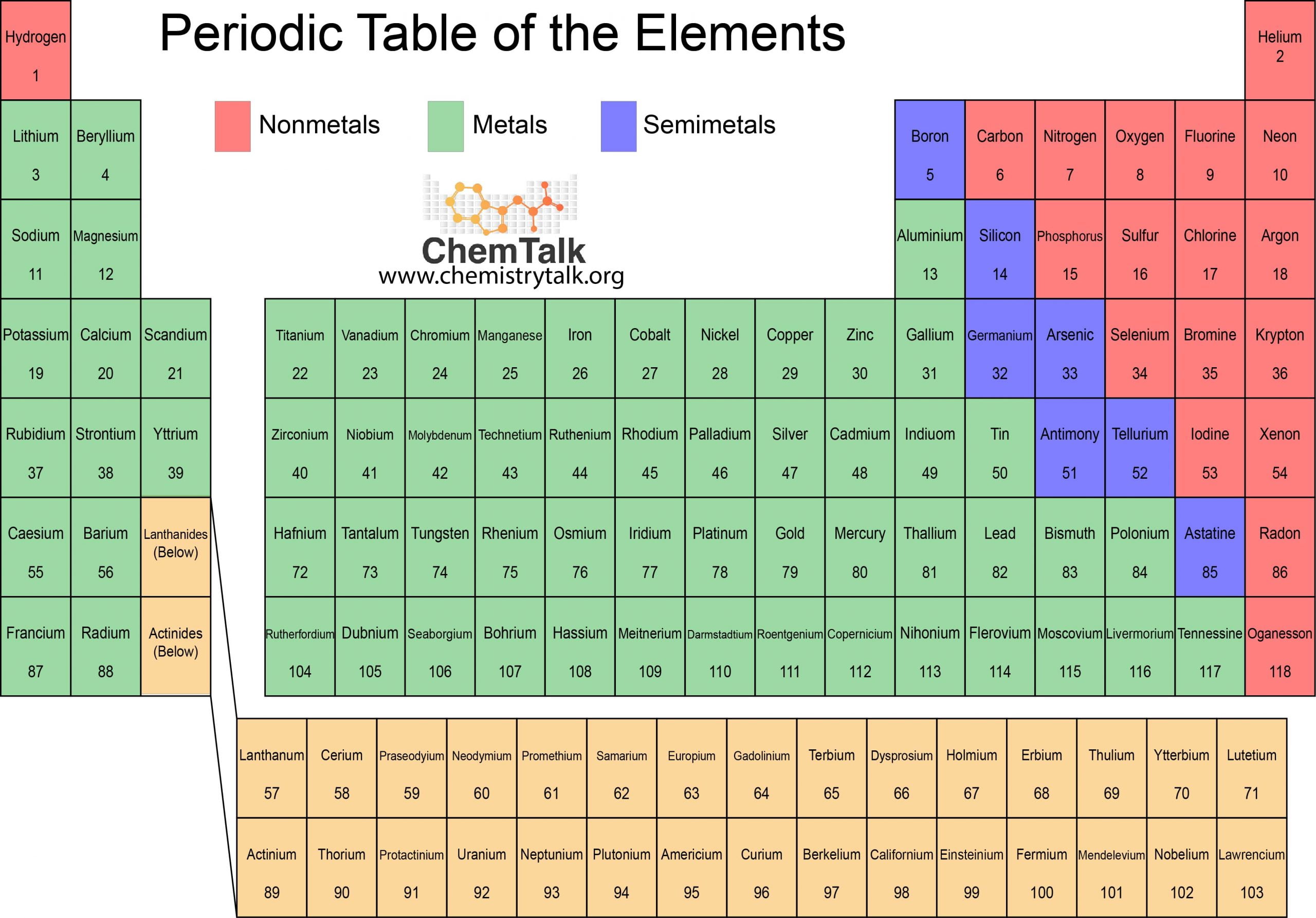
The elements in blue in the periodic table below are metals. Find out the differences between ferrous and non-ferrous metals, alkali and alkaline earth . Silver also has the highest thermal conductivity of any element and the highest light . Material selection is important because . They also lack magnetic properties.Introducing Meta Horizon OS. The atoms of metal elements are characterized by the presence of valence electrons, which are electrons in the outer shell of an atom that are free to move about. Properties of metals, metalloids and nonmetals. Uses of Metals. These oxides are alkaline in nature and have a high pH above 7 when dissolved in water.
Conductivity and Conductive Elements
The chemical elements can . Various properties of the group 1 elements are summarized in Table 20.
22: Metals
Most metals and alloys crystallize in one of three very common structures: body-centered cubic (bcc), hexagonal close packed (hcp), or cubic close packed (ccp, also called face centered cubic, fcc). Chemical properties of the metals include a tendency to lose electrons and form positive ions, and the ability of their oxides to function as bases.56 g/cm3 and platinum at 21. During a single-displacement reaction, a metal higher up the series will . Le rhénium est également inclus comme métal noble par certains scientifiques et ingénieurs. For example, copper melts at 1084°C and steel has a melting point that ranges from 1371°C to 1593°C.The elements of the periodic table can be broken into three different groups: Metals, Metalloids, and Nonmetals. We pause here to point out an .Les principaux métaux : Leur définition et usage. Other properties include: State: Metals are solids at room temperature with the exception of . account for around 20% of known.Like some metals, passivation of silicon occurs due the formation of a very thin film of oxide (primarily silicon dioxide, SiO 2).For any particular medium, a list can be made arranging metals sequentially from most active, or least noble, to passive, or most noble. Elements are further classified into metals, non-metals, . At the bottom are the least reactive metals. Find out which elements are alkali metals, alkaline earth metals, transition metals, . Generally, Steel, Titanium, Tungsten and Inconel are considered the strongest four metals.Metals are lustrous, malleable, ductile, good conductors of heat and electricity.Crystal structures. Very reactive metal ore is melted and electrolyzed using a DC .Auteur : Anne Marie Helmenstine, Ph. The relative size of each element in the mix plays a primary role in determining which mechanism will occur. Non-metals: gain electrons to form anions when reacting with metals .Celsius to Fahrenheit: (°C × 9/5) + 32. Silicon dioxide is soluble in hot aqueous base; thus, strong bases destroy the passivation.Learn about metal, a class of substances with high electrical and thermal conductivity, malleability, ductility, and reflectivity. Element 113 - Ununtrium - .1: Prelude to Metals.Les quatre principaux métaux précieux sont l'or, l'argent, le platine et le palladium. Element 13 - Aluminum. The most electrically conductive element is silver, followed by copper and gold. Element 81 - Thallium. It has a number of industrial and ornamental uses. Find a complete list of all metals with their names, properties, and . It is these free electrons that allow metals to conduct an electric current. This means that a significant amount of mass is concentrated in a small volume, resulting in high density. In 2011, 41 materials were assessed, 54 materials were assessed in 2014, and 78 in 2017.It’s actually used to measure the amount of energy the material can absorb at the limited state.20 Different Types Of Metal And Their Properties - .Properties of Metals . This new hardware ecosystem will run on Meta Horizon OS, the mixed reality operating system that powers our Meta Quest headsets. When the atoms are relatively similar in size, the . Removal of the passivation layer allows the base to dissolve the silicon, forming hydrogen gas and silicate anions.85 g/cm3, 304 stainless steel is 8. Notice that aluminum borders the line, but it is considered to be a . Elements to the left are metals and nonmetals are to the right. Frequently Asked . Element 82 - Lead.93 g/cm3, gold is 19.This table presents the electrical resistivity and electrical conductivity of several materials. Gold is one of the most popular precious metals in the world. La plupart des éléments sont des métaux. Technology and .Les métaux de base les plus courants sont le cuivre , le plomb , le nickel , l'étain, l'aluminium et le zinc. Medical Applications.Temps de Lecture Estimé: 3 min
Metal
Conductivity refers to the ability of a material to transmit energy.
Metals, Metalloids, and Nonmetals
Fahrenheit to Celsius: (°F − 32) × 5/9. About 80 percent of the . The melting point of mercury is -39°C, while the highest melting metal is tungsten (W), with a melting point of 3422°C. Element 50 - Tin. All metals can form oxides.Chemical properties.All metals are solid at room temperature with the exception of mercury (Hg), which is a liquid. Electrical resistivity, represented by the Greek letter ρ (rho), is a measure of how strongly a material opposes the flow of electric current.Learn about metals, their characteristics, and how they are classified based on their atomic number and symbol. Different metals have different densities, and the density difference between some metals is huge.

Metals are found on the left and in the middle, whereas .
Une liste de tous les éléments qui sont des métaux
In all three structures the coordination number of the metal atoms (i.This page lists metals, with subdivisions for alloys and specialised subsets of metal and metal-based compounds.com23 Different Types of Metals and Their Properties & Uses [PDF]theengineerspost. The exception is the element hydrogen.Find out the definition, properties, examples and history of metals and metalloids, and the alphabetical and periodic table of metals. Metals, shiny solids, are room temperature (except mercury, which is a shiny liquid element), with characteristic high melting points and densities. Refer to this chart for the melting points of various metals and alloys. This periodic table shows the three different groups of elements.List of Important Metals and their ores. Technological Applications.Learn the definition, properties, and examples of metals on the periodic table. It is also used in jewelry, car bodywork, dentistry, and . Voici un aperçu de ce qui rend un métal précieux par rapport à d'autres . Mis à jour le 03 février 2020. Safety Measures When Handling Metals.There are 118 elements known to us, out of which 92 are naturally occurring, while the rest have been prepared artificially.Cartridge brass, on the other hand, has a higher percentage of copper and is commonly used in ammunition casings and other applications requiring a stronger metal.Here's a list of all of the chemical elements of the periodic table ordered by increasing atomic number.
List of Metals to Rock Your Vocabulary
The density of common metals such as iron is 7.
List of All Elements Considered to Be Metals
On the periodic table, the elements colored yellow, which generally border the stair-step line, are considered to be metalloids.
Introducing Our Open Mixed Reality Ecosystem
The periodic table can be used to find out if an element is a metal or a non-metal.
Density of Metals
Alors que les métaux nobles conservent leur couleur brillante, les métaux de base ont tendance à s'oxyder à l'air humide. In keeping with overall periodic trends, the atomic and ionic radii increase smoothly from Li to Cs, and the first ionization energies decrease as the atoms become larger. These elements have metallic character, which means . Osmium is the heaviest known metal, with a density of 22.The reactivity series of metals is a list of metals arranged in their order of reactivity from highest to lowest. The galvanic series for sea water is discussed in the Chemistry Fundamentals Handbook.Non-metals: gain electrons to form anions when reacting with metals We pause here to point out an important exception to the statement that nonmetals tend to form anions.This is a list of elements that are basic metals.Yes, there are several metals that are heavier than gold.87 g/cm3, mild steel is 7. For example: \[\ce{Si}(s)+\ce{4OH .121 lignesLike the periodic table, the list below organizes the elements by the number of protons in their atoms; it can also be organized by other properties, such as atomic .When a metal has iron, it is called a ferrous metal. Element 49 - Indium.Electrical conductivity in metals is a result of the movement of electrically charged particles.
9 Nifty Metal stocks including Vedanta, NALCO, SAIL gained 20
Bronze is a non-ferrous metal alloy made by mixing copper with other metals such as tin, zinc, or lead.
Electrical Conductivity of Metals
Learn about the 94 metals in the periodic table, their properties, and how they are used in various fields. Les métaux de base sont plus courants et plus facilement extraits .Pure metals tend to have relatively high densities because their atomic structure is closely packed. The so-called inert gases (sometimes called the Noble Gases), are a family of elements that neither gains nor loses electrons easily; they comprise Group 8A on the extreme right . When a molten metal is mixed with another substance, there are two mechanisms that can cause an alloy to form: (1) atom exchange or (2) interstitial mechanism. Melting points of metals display a very wide variance.The 2020 criticality assessment was carried out for 66 candidate materials (63 individual materials and 3 material groups: heavy rare earth elements, light rare earth elements, platinum group metals, amounting to 83 materials in total). High molecular weights: Metals have a high atomic number and also atomic weight.Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. For example, the most dense metal is Osmium (Os), with a density .A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. In contrast, ceramic oxides such as Al 2 O 3 cannot react with oxygen regardless of the temperature because aluminum is already in its highest . At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions.Copper, manganese, and zinc round out the top five industrial metals mined in 2021, each with its own unique properties and roles in the economy. Element 83 - Bismuth. Celsius to Kelvin: °C + 273.
Find out the difference between ferrous and non-ferrous .

Several borderline cases such as B, Si, Ge, As, Sb, and Te are difficult to classify as metals or .Few metals can be used in jet engines, for example, because most lose mechanical strength and react with oxygen at the very high operating temperatures inside the engines (approximately 2000°C).