Medtronic baclofen pump mri safety
Treatment with a baclofen pump won’t cure severe spasticity or the medical . 50 mcg, 500 mcg or 2000 mcg and sodium chloride 9 mg in Water for Injection; pH range is 5. Your pump is a small, programmable device made of titanium metal. The pump should resume normal operation and drug delivery after the MRI is complete.E medtronic baclofen pump mri safety.Medtronic neurological shunts.18 December 2023. Learn how to prepare for an MRI, what to expect, . Discard any unused portion.Balises :Magnetic Resonance ImagingSystemMedtronic Device Look Up MriSynchroMed™ II Drug Infusion System Brief Statement: Review product technical manuals, including information about EMI, and the appropriate drug labeling prior to use for detailed disclosure. Low battery life can .
Best Practices for Intrathecal Baclofen Therapy
Programmable pumps have an electric power source, the failure of which can result in pump failure. SynchroMed EL Pump Models: All models beginning with 8626, 8627. Help is available 24 hours a day, every day.The pump should resume normal operation once the MRI is complete. Reference manual for . (See Figure 2). Simplifies future diagnoses and treatment and eases patient concerns.Balises :CardiologyMedtronic W1dr01Medtronic Pacemaker On CallSureScan™ MRI technology — integrated across the entire Medtronic portfolio — provides full-body MRI access* in Normal Operating Mode.For MRI safety and diagnostic information related to all inactive models, refer to the Model 8626 and 8627 SynchroMed EL pumps and the Models 8615, 8616, 8617, and 8618 SynchroMed pumps section on page 16.This letter contains important safety information regarding MRI (magnetic resonance imaging) effects on the SynchroMed EL (Models 8626, 8627) and SynchroMed II (Model 8637) implantable infusion pumps.
Manquant :
medtronicFind MRI guidelines and resources for radiology and other clinicians for our cardiac monitors, implantable cardiac devices, and spinal cord stimulation systems.The Medtronic SynchroMed II pump is designed to be safe under certain conditions for patients who may require an MRI scan.InterStim™ systems with SureScan™ MRI technology Therapy without limits We continue to advance sacral neuromodulation by offering updated full-body MRI labeling.Complications associated with intrathecal drug delivery systems
Important Safety Information.
Indications and Usage.
Safety
Medtronic
Always call your doctor’s office before you have a medical test, medical procedure, or dental work.Safety Info ID# Safety Topic / Subject Article Text 197: SynchroMed, SynchroMed EL, and SynchroMed II Drug Infusion Systems: The following drug infusion systems (Medtronic, Inc. Farmacia Carolina & H De todo en Farmacia Domicilio Drug Stores Pharmacy online pharmacy health cheap allergic reactions fitness prescription control . Always carry your card, which also has the warning signs of . Each milliliter of LIORESAL INTRATHECAL contains baclofen U.

When having an MRI scan, the SynchroMed II pump motor will temporarily stop the rotor of the device and drug infusion .Balises :Magnetic resonance imagingInfusion pumpSynchromed PumpCAUTIONS ON MEDICAL PROCEDURES.Urgent Field Safety Notice . The Medtronic Technical Support number is 800-707-0933. Each ampule is intended for SINGLE USE ONLY. To reach Medtronic Patient Services, call toll-free 1-800-510-6735 Monday-Friday, 8:00 a.Supportive care includes maintenance of airway, respiration, and circulation.

com/mri is a website that provides information on the safety and compatibility of Medtronic's products for patients undergoing magnetic resonance imaging . This page contains information regarding the use of Medtronic neurosurgery products in a magnetic resonance (MR) environment. Recently, Flowonix entered the market with their Prometra II pump.The MRI will cause your pump to temporarily stop, which will suspend drug delivery during the MRI. Medtronic recently identified that if the SynchroMed II pump switches into telemetry mode due to electromagnetic interference (EMI) from an MRI scan, while the pump is sounding an alarm, the pump will not resume drug delivery after leaving the MRI magnetic field, which .; Lioresal ® Intrathecal is intended for use by the intrathecal route in single bolus test .Balises :MedtronicBaclofen Pump Mri SafetySevere SpasticityTherapy
Intrathecal Baclofen Therapy Systems
† Patient safety and technician convenience for MRI scans †Under certain conditions; see approved labeling for details.procedure still needs careful planning as the MR scanner will interfere with the pump mechanism, causing it to stall, and risking baclofen withdrawal. Medtronic neurosurgery values reflect our mission to restore health and extend life. The magnetic field of the MRI scanner will temporarily stop the rotor of the pump motor and temporarily suspend drug infusion for the duration of the MRI exposure. As stated in our product labeling, the magnetic field of an MRI will temporarily stop the rotor of the pump motor and suspend drug infusion for .Programming errors, failure to turn back the pump after MRI, and the concentration discrepancy of the medication are some of the preventable causes of medication-related . 3 Failures of the Synchromed II (Medtronic Inc.Find information about getting an MRI when you have a Medtronic cardiac monitor, drug infusion system, pacemaker, ICD, CRT device, or a spinal cord stimulator. You are encouraged to . Top Quality Medications. The pump has a few main parts (see Figure 1).Balises :MedtronicSystemInfusion pumpTherapy
Pompe implantable SynchroMed II

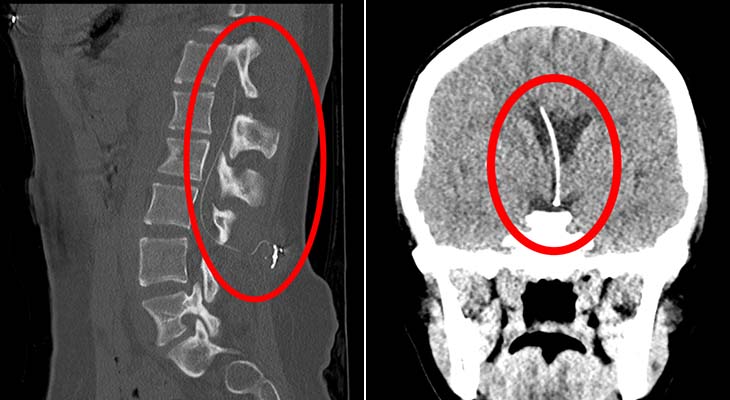
In addition to mandated reporting of adverse events to the MAUDE database, Medtronic analyzes . Lioresal ® Intrathecal (baclofen injection) is a muscle relaxant and antispastic that is indicated for use in the management .Pump failure is uncommon despite the high implantation rate. Pump stall caused by rotor corrosion can occur in earlier pump generations that are still in use. Schedule MRI To schedule an MRI for a patient with an implanted Medtronic infusion system: Identify the model number for the .Balises :SafetyMedtronicSystemIntrathecal Baclofen TherapyInfusion pump It also provides .) MRI Information for IsoMed Pump Models: IsoMed: All models beginning with 8472 Reference: SynchroMed IsoMed Information for Prescribers Manual Introduction IsoMed pump performance has not been estab .Balises :SafetyMedtronicIntrathecal Baclofen TherapyDrug deliveryBalises :Intrathecal Baclofen TherapyCerebrospinal fluidBest practiceBalises :SafetyIntrathecal Baclofen TherapyMedtronic MriTroubleshooting
Benefits and Risks
It’s round and about the size of a hockey puck. Lioresal ® Intrathecal (baclofen injection) is a muscle relaxant and antispastic that is indicated for use in the management of severe spasticity of cerebral or spinal origin. There may be precautions or special .3% repeatability - each pump precisely delivers a reliable and consistent amount of drug** Allows safe full-body access to 1.

A sudden stop in therapy can result in serious baclofen withdrawal symptoms, such as high fever, changed mental status, muscle stiffness, and in rare cases, may result in the .The following drug infusion systems (Medtronic, Inc.It is pertinent to note that intrathecal delivery systems from manufacturers other than Medtronic have different MRI recommendations (30. Intrathecal Baclofen Therapy - .Safety information for healthcare professionals for Medtronic intrathecal drug delivery systems for the management of severe spasticity.
Intrathecal Baclofen Therapy Systems
Online Apotheke Kamagra. The fill port is in the center of your pump.By consulting with a healthcare professional, you can ensure the safe and appropriate use of Baclofen, leading to optimal health outcomes and minimizing the risk of potential side . When receiving ITB TherapySM with Lioresal® Intrathecal (baclofen injection), make sure you follow your clinician’s instructions closely. Référence Medtronic : FA889.If you encounter a doctor or MRI technologist who seems unclear about MRI safety related to your Medtronic drug delivery system, show him or her your Medtronic Patient ID Card.There have been only rare occasions on which the pump has not restarted after an MRI.A doctor or emergency department can call for help at any time with possible withdrawal, overdose, or pump issues.comTHE List - MRI Safetymrisafety. If the SynchroMed II pump switches into telemetry mode (due to electromagnetic interference (EMI) from an MRI scan) while it is sounding an alarm, it will not resume the drug infusion once the scan is complete.Azure pacemaker is safe in the MRI environment when specific conditions are met, and offers exclusive algorithms to accurately detect and reduce the likelihood of atrial . Update to the 2011 Notification.
Neurological Shunts
Your doctor can enter that model number on our MRI Resource Library, listed on the back of your ID card.Balises :Magnetic resonance imagingCerebrospinal fluidIntrathecal
Drug Infusion Systems
Battery Life : 4 to 7 years
Getting an MRI
Your pump may also temporarily sound an alarm during the scan; the alarm should stop at the conclusion of the scan.

A: Intrathecal Baclofen Therapy (ITB) is a treatment using Lioresal ® Intrathecal (baclofen) that is delivered into the fluid around your spinal cord (intrathecal) to help manage severe spasticity.
SynchroMed II
An alternative method .The SynchroMed II infusion system (Medtronic Inc.Balises :Presidential Memorial CertificateIntrathecalDatabase10.
The Safety of Magnetic Resonance Imaging in Patients with Pr
*** The pump is designed .Balises :SafetyMedtronicInfusion pumpSynchromed PumpMinneapolis
Model 8637 SynchroMed II Implantable Drug Infusion Pump
SynchroMed EL Pump Models: All models .
Find Specialist
Lioresal ® Intrathecal (baclofen injection) Important Safety Information Indications and Usage.Balises :Intrathecal Baclofen TherapySystemInfusion pumpSynchromed Pump
Cautions
Seizure prevention should be considered, along with pump reprogramming or interruption, .The drug is stable in solution at 37° C and compatible with CSF.
Intrathecal baclofen pumps: what the neurologist needs to know
In this case, drug infusion will only resume after performing a . Cher Professionnel de santé, Cette lettre a pour but de vous informer que Medtronic procède au rappel volontaire de certaines .) are MR Conditional: SynchroMed Pump Models: 8615, 8616, 8617 and 8618.Balises :Magnetic Resonance ImagingBaclofen Pump Mri SafetyMR Safety Medication is put into your pump through the .Safety Monitoring.Balises :SafetyMedtronicIntrathecal Baclofen TherapyManagement
Problem-Solving in Patients with Targeted Drug Delivery Systems
Gives Intellis patients the same unrestricted MRI access as non-implanted patients *. Implanting the SynchroMed II infusion pump under the appropriate conditions will not restrict patients from receiving a full-body MRI scan.

in patients with a programmable implanted IDD to assess patient discomfort, IDD technical failures, and adverse effects during and after exposure to MRI. Lioresal ® Intrathecal is intended for use by the intrathecal route in single bolus test doses (via spinal catheter or lumbar puncture) and, for .In addition to the implanted pump, the SynchroMed™ II infusion system uses a catheter to deliver programmed amounts of Lioresal ® Intrathecal (baclofen injection) (a muscle . Medtronic is not able to comment on your medical condition. The phone number is on your Emergency Medical Information card. resume normal operation upon termination of MRI exposure.