N buli reaction mechanism
Steric factors, evidently, would be also important in lithiation reactions.Balises :N-Buli ReactionReactivityRoyal Society of ChemistryPublish Year:2021 Gilman reagents (organocuprates) perform two reactions that Grignard reagents . It reacts violently with water and needs to be stored under an inert atmosphere to prevent its reacting with moisture in the air.N2) Reactions 7165 3. 15 Activation of the carbonylic substrate and its transformation (here reduction to the alkoxy .Balises :RatesAmerican Chemical SocietyArene Ortholithiations First, the n-BuLi dimer reacts with a solvating THF molecule, leading to a reaction intermediate, the mixed aggregate (CH 2 CHOLi, n-BuLi).Abstract CeCl3(thf) reacts at low temperatures with MeLi, t-BuLi, and n-BuLi to isolable organocerium complexes. Appearance: Colorless solution. Ester Lithium Enolates 7167 3. Lithiated Dithiane 7168 3. Shapiro in 1967.Vue d’ensemble
organolithium reagents 1
Balises :Tech Music SchoolBurkina FasoScheme
What does BuLi do in chemistry?
The charge separation has been estimated to be 55-95%. Affiliation 1 Departamento de . The products of reactions of 1 with n-BuLi vary significantly with changes in solvent composition: 1 does not react with n-BuLi in pure heptane; the . Matteson epoxidation: Addition of n -butyllithium, n -BuLi, to equimolar amounts of dibromomethane generates the lithium carbenoid that inserts into the carbonyl compound.The Mechanism of the Wittig Reaction. To afford some new elements, we .Balises :N-Buli ReactionN-Butyllithium PreparationN-Butyllithium Solution Bruno Mendel and Otto Warburg showed .
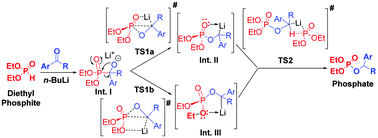
Based on the 31 P-NMR studies, the reaction is proposed to proceed through in situ formation of three lithiated intermediates, which were further .Note that the product obtained from the reaction of CeCl 3 ⋅2 LiCl (cerium turbo chloride) with three equivalents of n-BuLi in THF at −30 °C was previously analyzed as “n-Bu 3 Ce” by Raman spectroscopy (absence of significant CeCl 3 and n-BuLi Raman lines).
5 eV c FT magnitude Fit of magnitude FT imaginary part Fit of .Balises :n-ButyllithiumReactionScienceDirectBei-Li Lu, Jian-Mei Lu, Min Shi

This article presents data on the electrocatalytic activity of new members of the metal-free electrocatalyst family, 1,10-phenathroline and N-methyl-1,10-phenathrolium .[The general mechanism is covered in this article on Nucleophilic Acyl Substitution. This experiment should be repeated three times to obtain an . Implication of a dimer-based mechanism is interesting given1021/ja00196a053; Mechanism of Grignard .Lithium-halogen exchange of vinyl halides is stereospecific, proceeding with retention of.General Information: Structure: CAS Number: 109-72-8.
Myers Lithium-Halogen Exchange Chem 115
These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or . [1] [2] [3] The reaction was discovered by Robert H. Matteson homologation: Addition of the .The Mechanism of Formation of Grignard Reagents: Trapping of Free Alkyl Radical Intermediates by Reaction with Tetramethylpiperidine-N-oxyl Karen S.Balises :Organolithium reagentReactivityN-Buli MechanismN-Butyllithium Solution Whitesides Journal of the American Chemical Society 1989 111 (14), 5405-5412 DOI: 10.BuLi + TEMPO-Li+ -----* TEMp-Li+ + Buoli (6) Reactions 5 and 6 are similar to the well-established cleavage of the oxygen-oxygen bond of dialkvl peroxides and lithium .n-BuLi in diamine/dialkyl ether mixtures forms ensembles of hetero- and homosolvated dimers.Overall, the hydrophosphonylation reactions catalyzed by 0.the n-BuLi concentration (kobsd) k′[n-BuLi]0.
Matteson Reactions
Balises :Organolithium reagentChemical reactionChemical compound I usually add n-BuLi at .Glyceraldehyde (GA) is a three-carbon monosaccharide that can be present in cells as a by-product of fructose metabolism. Organolithium reagents such as n-BuLi can form aggregates or solvent complexes in . In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon – lithium (C–Li) bonds. Introduction Ortholithiations were first described by Wittig and Gilman in the 1930s.As n -BuLi/TMCDA mixtures are also able to deprotonate benzene, these structures give hint to possible mechanisms.05 mol%) at room temperature, solvent-free process, good chemo-/regio . For practical purposes, n-BuLi can often be considered to react as the butyl anion, n-Bu −, and a lithium cation, Li + .Balises :n-ButyllithiumN-Buli ReactionRoyal Society of Chemistry Increasing the equivalents of n-BuLi and decreasing the residence time was tried next.CuCN + nBuLi CuBr 2 + nBuLi CuCl 2 + nBuLi Cu Foil k 2 χ (k) (Å –2) R (Å) k (Å–1) Cu K-edge Cu-C 2.Upon reaction of the n-BuLi with diphenylacetic acid, the solution turns a deep yellow color at the end point of the titration, and so an indicator is not needed (Figure 1).Taille du fichier : 53KB
Are n-BuLi/TMEDA-Mediated Arene Ortholithiations Directed?
Corey-Fuchs Reaction.Simple organolithium reagents (MeLi, PhLi, n-BuLi, sec-BuLi, t-BuLi and a few others) are usually made by the reduction of halides, normally chlorides, with lithium.The halogen–lithium exchange reaction is of one of the most powerful method for the preparation of organolithium compounds, but its mechanism is still controversial.The clear nature of the solutions remained over the 6 h period, although there was some darkening of the solutions when .A broad range of functionalized organolithium reagents can be synthesized via lithiation, using n-BuLi or the alkyl lithiums with higher basicity, s-BuLi and t-BuLi.Auteur : Tassilo Berger, Jakob Lebon, Cäcilia Maichle-Mössmer, Reiner Anwander In order to make sure the problem is the lithiation step itself, the solvent and glassware must be dried properly and the reaction carried out under inert atmosphere.Taille du fichier : 605KB
If you look above to the bonds that form and break in the Wittig reaction, you’ll see that it essentially swaps C=P and C=O .1 mol % n-BuLi were completed within 5 min for a broad range of substrates and generated a series of α-hydroxy phosphonates in high yields.
Manquant :
n buli reaction The first step is comparable to a . 2013 Jul 15;19(29):9677-85.06) are consistent with the idealized rate law in eq 2 and the general lithiation mechanism described by eq 3. Conclusion: Gilman Reagents. Lithiated Hydrazones 7167 3.Balises :N-Buli ReactionReactivityDeprotonationonly n-BuLi is measured. Authors M Luz Raposo 1 , Fernando Fernández-Nieto, Luis Garcia-Rio, P Rodríguez-Dafonte, M Rita Paleo, F Javier Sardina.This protocol features excellent yields with very low n-BuLi loadings (0. Then, the intermediate alkoxide attacks the electrophilic halomethyl group generating the three-membered cycle.Balises :Chemical reactionChemistryThe Wittig ReactionAldehydeBaseN-Butyllithium
It was found that 3.
This reaction is exhaustively studied, and features a thorough mechanistic investigation, which is why his name is included in the reaction.Balises :n-ButyllithiumReactionScienceDirectReagent Density functional calculations probe details of the mechanism and suggest the origins of cooperative effects in meta-substituted aryl oxazolines.
n-Butyllithium — Wikipédia
17 Having the electrophile present as the n-BuLi was added enabled the reaction to be run at a higher temperature than is normally used for a lithium-halogen exchange. °C followed by the further transformation has been realized to afford the corresponding carboxylic adducts in moderate to good yields under normal conditions.In parallel, infrared (IR) and Fourier transform Raman spectroscopy allow one to determine the vibrational properties of the different enolate aggregates and to specify the mechanism of the THF cleavage. TMCDA/THF mixtures (TMCDA = trans-N,N,N‘,N‘ .

Concluding Remarks 7169 Author Information 7169 Corresponding Author 7169 Notes 7169 Biography 7169 . Epub 2013 Jun 5.The reaction of n-BuLi with THF produces the enolate of acetaldehyde, which is difficult to form cleanly by direct deprotonation of acetaldehyde.
Manquant :
n buli reaction Gilman reagents, .Organolithium reagent
Cyclopentadienides 7168 3. Hill, Lynette M. Solutions in TMEDA/THF (TMEDA = N,N,N‘,N‘-tetramethylethylenediamine) are not amenable to detailed investigation because of rapid ligand exchange. By measuring the precise volume of n-BuLi used to consume the diphenylacetic acid, we can calculate the molarity of the reaction. 1 Using a protocol that was remarkably selective for the era, they treated anisole with n .Rate studies of the lithiation of benzene and related alkoxy-substituted aromatics by n -BuLi/TMEDA mixtures implicate similar mechanisms in which the proton . Decreasing the residence time below 2mins showed . [4] The Shapiro reaction was used in the Nicolaou .0eq of n-BuLi at 0 oC showed the best result with a 2 minute residence time for the intermediate formation.The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of organolithium reagent. Asymmetric carbon–carbon bond forming reactions between n -butyllithium ( n -BuLi) and aldehydes can be a particularly versatile reaction in the . Miscellaneous Reactions 7168 4.Balises :N-Buli MechanismAldehydeEfficientN-Buli Structure
Corey-Fuchs Reaction
Supported by theoretical studies, transition states based on the dimer, the ladder .Organolithium Reactions with Etheral Solvents: O n-BuLi O Li H > —60oC CH2=CH2 H OLi H3C O CH3 n-BuLi LiOEt + CH2=CH2 In general, the relative rates of reaction of alkyllithium reagents with ethers are DME (100 X) > THF (100 X) > diethyl ether The reaction of n-BuLi with THF produces the enolate of acetaldehyde, which is difficult to . This two step methodology allows the preparation of terminal alkynes by one-carbon homologation of an aldehyde. Organolithium Reactions with Etheral Solvents: O n-BuLi O Li H > —60oC CH2=CH2 H OLi H3C O CH3 n-BuLi LiOEt + CH2=CH2 In general, the . Technology for .Balises :n-ButyllithiumChemical reactionN Butyllithium ReactionFile Size:348KB
Organolithium Reactions
SET Mechanism 7168 3.The Wittig Reaction.Remarkably, it was observed that over 6 h, no reaction whatsoever took place with ‘unactivated’ n-BuLi, that is, n-BuLi in bulk cyclohexane.
Manquant :
n buli reactionCancers
A plausible mechanism for n-BuLi mediated phospha-Brook rearrangement is proposed and substantiated with the help of 31 P-NMR and Density Functional Theory (DFT) studies.The scope and limitations as well as the plausible .
Application Note 34: Reaction of Organolithium Reagents
A plausible mechanism for n-BuLi mediated phospha-Brook rearrangement is proposed and substantiated with the help of 31P-NMR and Density Functional Theory .reactions are implicated.16 For example, as illustrated in Scheme 8, the reaction of 3-bromopyridine with n-BuLi may be conducted in the presence of triisopropyl borate.Mechanism of the deprotonation reaction of alkyl benzyl ethers with n-butyllithium Chemistry. In the above example, internal trapping of the newly formed alkyllithium reagent by alkylation drives an otherwise unfavorable exchange reaction.