New drug list
New Generic Drug Approvals for 2024
FDA new drug approvals in Q3 2021.Nature Biotechnology - New drug approvals reached an all-time high in 2023, with five gene therapies, the first CRISPR–Cas9-edited therapy and a disease . This drug is used along with exercise and diet to improve glycemic control in adults with type 2 diabetes. To note: All products added to the list in April 2024 are shown in red font.Subsection 505(t)(3) defines an “authorized generic drug” as a listed drug as defined in § 314. Monthly lists of medicines under . Link to the list of drugs preferred by MassHealth based on supplemental rebate agreements between MassHealth and drug manufacturers.The current versions, updated in July 2023, are the 23 rd Essential Medicines List (EML) and the 9 th Essential Medicines List for Children (EMLc).
What's new
SUGAM - An e-Governance solution for CDSCO.
The Newest Diabetes Drugs on the Market
2021 FDA approvals. You can view each condition and its drug list, last updated September 2021. What's new - Menu tabs Current month Previous month Two months ago. The NDA application is the vehicle through which drug sponsors formally .An obesity drug called Zepbound won approval for use in adults from the Food and Drug Administration Wednesday, ushering in a new rival to Novo Nordisk's blockbuster Wegovy. Out of the 37 novel drug products approved by the FDA in 2022, 22 were small molecule drugs (59%), and 15 were biologic drugs (41%).
FDA new drug approvals in Q3 2021
MassHealth Supplemental Rebate/Preferred Drug List.To see the FDA-approved conditions of use [e.
List of new drugs approved in the year 2021 till date
What's new: Drug products
How HIV Is Treated. The Anthem Blue Cross and Blue Shield National drug list is for New York members who receive a health insurance plan from an employer, if they have certain grandfathered plans, or in some cases if coverage is through a Small Group employer. Consult the Drug and Health Product Register regularly for Regulatory Decision . About this PDL Where differences exist between this PDL and your benefit plan documents, the benefit plan documents rule.All the new drugs approved in 2023: Alzheimer's and gene therapy cap a five-year high. Business appears to be back to normal at the FDA. WHO Electronic .List of medicinal products under additional monitoring. National Essential Medicine List (5th edition, 2014) 9. Members, contact your employer or the Pharmacy Member Services number on your ID . The biggest FDA approval of the third quarter was for a product that has already been used in more than 130 countries. When it comes to innovation in the development of . The FDA’s Center for Drug Evaluation and Research (CDER) approved 55 new drugs in 2023, as the small molecule and biologic pharmacopoeia . Browse medications alphabetically. The FDA approved 50 novel drugs in 2021, including the first KRAS inhibitor for cancer and the first anti ., indication (s), population (s), dosing regimen (s)] for each of these products, see the most recent A-approved Prescribing .04 January 2022.
New York Drug List
Thirty-seven of the 50 (74%) novel drug .
Human medicines: highlights of 2022
Advanced Service Specification – NHS New Medicine Service (NMS) [PDF: 245KB]As outlined in Part VIC of the drug tariff, you should only prescribe NMS to patients who’ve been prescribed a particular NMS medicine for the first time and for the following conditions. Current estimates for research and . Select your medications. Access the world’s pharmaceutical knowledge database.No Name of drug Indication Date of issue 1 Triamcinolone Hexacetonide injectable suspension 20mg/ml For intraarticular, intra-synovial or periarticular use in adults and adolescents for the symptomatic treatment of subacute and chronic inflammatory joint diseases including rheumatoid arthritis and . The table and chart below compare the figures over the last 11 years (2012-2022).Our drug lists include details about brands and generics, dosage/strength options, and information about prior authorization of your drug. Approved Drugs Expanded for New Pediatric Populations.2022 FDA approvals.Some of the most common ones, which can emerge within days of starting the medication, are a decrease in libido, headache, dry mouth and upset stomach. This listing does not contain vaccines, allergenic products, . New Drug Review with Electronic Data; Biosimilar; Regulations and Approval/Certification of Medical Devices; Regenerative Medical Products; Advanced Efforts. Here, we’ll review nine of the most influential drug and vaccine approvals of 2023, all selected by GoodRx Health pharmacists.
Novel Drug Approvals for 2023
The first Essential Medicines List was published in 1977, and the first Essential Medicines List for Children was published in 2007.
This PDL is not a complete list of .List of new drugs approved in the year 2023 till date. 2023 was a strong year for innovative new drugs, with new medications for Alzheimer’s disease, weight loss, and the first treatment based on the . Sorin Avram, Liliana Halip, Ramona Curpan & Tudor I.For decades, the regulation and control of new drugs in the United States has been based on the New Drug Application (NDA). The current versions, updated in July 2023, are the 23 rd .Notice regarding New Drugs dated 18., indication (s), population (s), dosing regimen (s)] for each of these products, see the most recent A-approved Prescribing Information (click on the.
DrugBank Online
Omidenepag isopropyl ophthalmic solution 0.
Novel Drug Approvals for 2021
In general, MassHealth requires a trial of the preferred drug or clinical rationale for prescribing a non-preferred drug within a therapeutic class. All products removed from the list are shown with .
New FDA Drug Approvals for 2024
Glyxambi (empagliflozin and linagliptin) was approved in 2015.Novel Drug Approvals for 2020. DirectorateGeneral Of Health Services.2020: 2020-Feb-18: 943 KB: 9: Notice regarding the FAQs on New Drugs and Clinical Trial dated 18/2/2020: 2020-Feb-18: 893 KB: 10: Additional FAQ on New Drugs and Clinical Trial Rules, 2019: 2019-Aug-23: 152 KB: 11: Frequently Asked Questions (FAQs) on New Drugs and Clinical Trials: 2019-Apr-26: 478 . In the second quarter of 2023, the agency approved 13 new drugs (Table 1), equalling the first quarter tally .New Drug Therapy Approvals 2022Novel Drug Approvals for 2021Novel Drug Approvals for 2020
Fresh from the biotech pipeline: record-breaking FDA approvals
002% w/v For the treatment of glaucoma and ocular hypertension 12. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of use (W HO/EMP/IAU/2019. In 2022, the number of novel drugs approved in the USA, European .While several new antiretroviral drugs have been added to the treatment arsenal since 2010, older ones like Crixivan (indinavir), Invirase (saquinavir), Rescriptor (delavirdine), Videx (didanosine), Viracept (nelfinavir), and Zerit (stavudine) have been discontinued and are no longer in use. Weekly Drug NOC updates.
Medicines for human use under evaluation
Filters are available to narrow results down based on your interests, . Ministry of Health &Family Welfare, Government of India. S pending on GLP-1 drugs like Ozempic and Wegovy ballooned last year and they’re set .
Eli Lilly & Co.But due to the nature of a medication’s expected impact, some new FDA approvals represent especially meaningful steps forward.List of new drugs approved in the year 2022 till date S.
New Drugs List 2021& 2022 approved by CDSCO
New Uses of Approved Drugs. List of new drugs . Below is a listing of new molecular entities and new . COVID-19 medicines; The CHMP meets once per month.Auteur : Asher Mullard
2021 FDA approvals
January 4, 2024 2:20 PM EST. Eisai, which is leading on Leqembi’s launch, had set a target of getting 10,000 US patients on to the therapy by the end of .First-Time Generics are the very first generic versions of marketed brand-name drug products to be approved by the FDA. Information on drugs, drug targets, and more, used by researchers and health professionals globally.3 that has been approved under subsection 505(c) of the act and is marketed, sold, or .
Avanafil bulk and Avanafil tablets 50mg/100mg/200mg For the treatment of erectile dysfunction 26.
Antidepressants: What to Know About Uses and Side Effects
Dosage may then be increased to 25 mg empagliflozin/5 mg .List of new drugs approved in the year 2021 till date.FDA new drug approvals in Q2 2023.
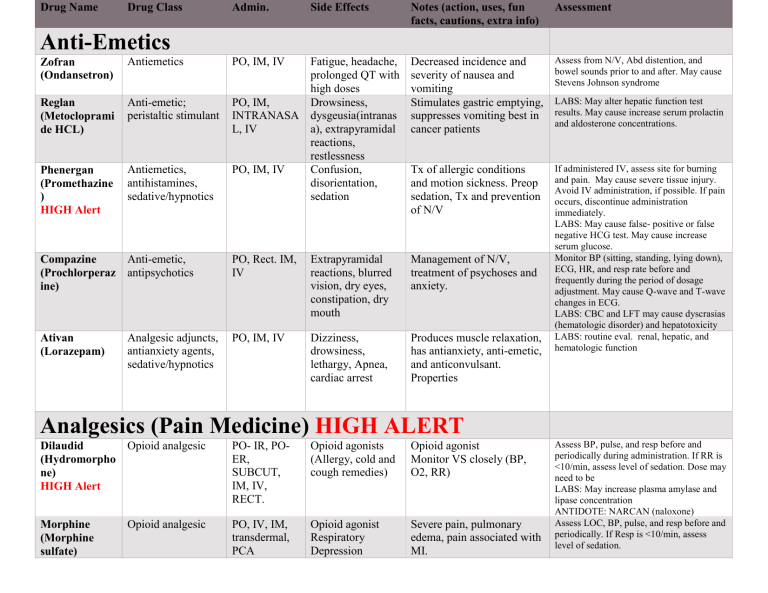
Files added to the Web site are also posted here in the What's new section for three weeks.
See drug prices.Press Release
Novel Drug Approvals for 2023
Keep in mind: The recommended dose is 10 mg empagliflozin/5 mg linagliptin once in the morning.For information on treatments and vaccines for COVID-19, including those under evaluation by the CHMP:. Its evaluations of marketing authorisation applications submitted through the centralised procedure provide the basis for authorisation of medicines in Europe.The WHO Model Lists of Essential Medicines are updated every two years by the Expert Committee on Selection and Use of Essential Medicines. Update 19 January 2022.Create a free account today! Sign up to unlock full drug cards and to stay up-to-date on product drops, features, and new data library packages.More News Resources
2023 FDA approvals
This overview covers up to two months back.
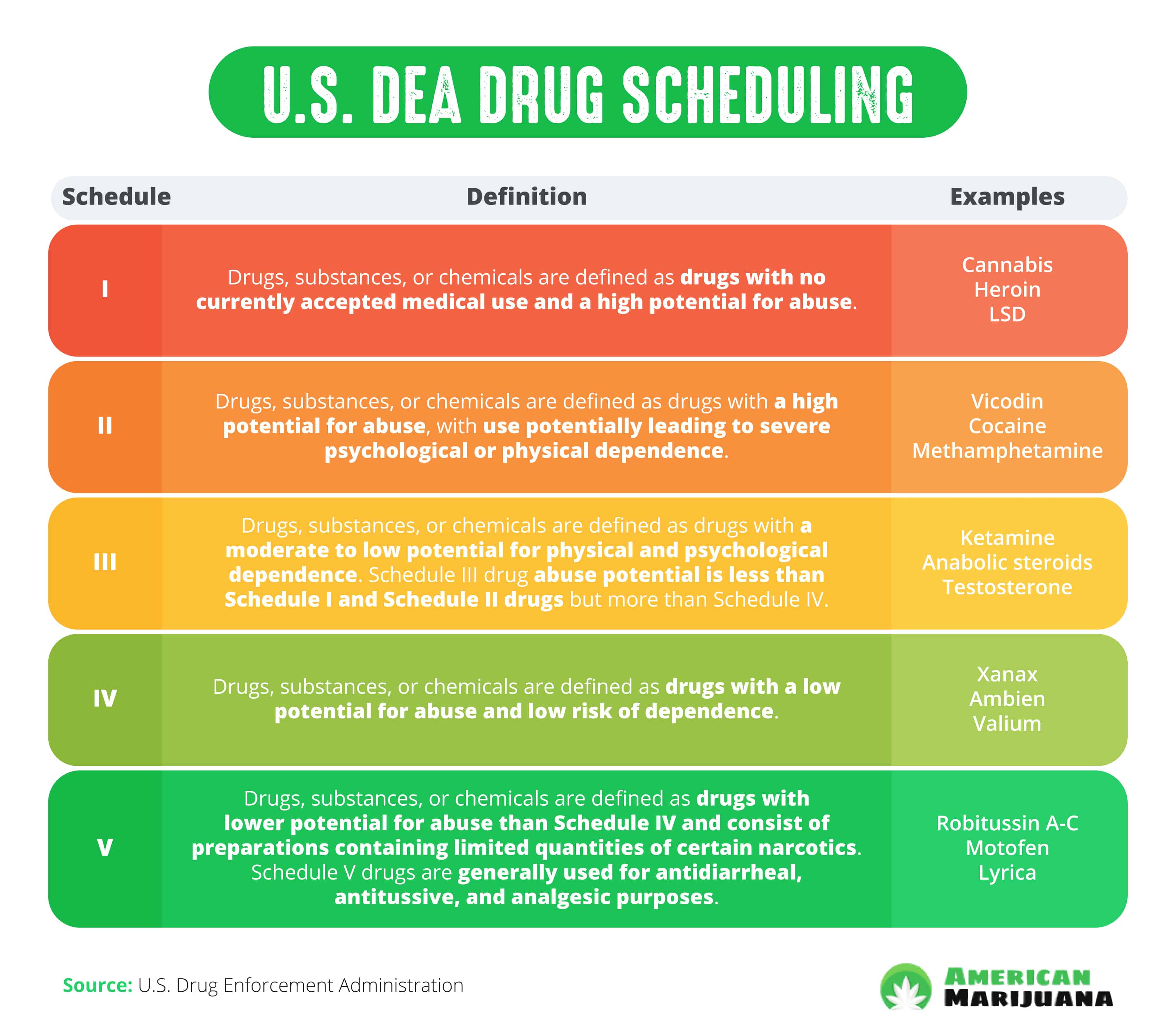
No Name of drug Indication Date of issue 1.

Strategy of SAKIGAKE by MHLW; Information for Approved Products. How We Choose The Drugs On These Lists An independent group of practicing doctors, pharmacists and other health-care professionals meet regularly to review new and existing drugs. Once you have browsed through the entire site, all you need to do to keep current is make sure you visit this page more than once every three weeks.This means that the FDA’s approval of the new use for Wegovy potentially opens up access to this drug for 1 in 4 people on Medicare with obesity or overweight. But keep in mind this isn’t an exhaustive list. Innovation drives progress. August saw the full approval of Pfizer and .National Drug List.
New Drug Application (NDA)
, indication (s), population (s), dosing regimen (s)] for each of these products, see the most recent FDA-approved .The Human medicines highlights 2022 of the 2022 key recommendations published today includes figures on the authorisation of medicines and a selection of . Central Drugs Standard Control Organisation.