Oral thrombin inhibitor
As a library, NLM provides access to scientific .5 nmol/L), by hydrolytic cleavage mediated by plasma esterases in vivo.Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of pulmonary embolism. Recent prospective randomized trials have shown the efficacy of dabigatran for the prevention of venous thromboembolism after knee or hip arthroplasty and for the prevention of stroke and systemic embolism in nonvalvular atrial . Healthy volunteers (n = 23) received 150 mg dabigatran etexilate twice daily on days 1 to 3 and once on day 4 in 1 period.
Direct Oral Anticoagulants: A Quick Guide
New oral anticoagulants (NOAc) have been shown to be not inferior to vitamin K antagonists in reducing thrombo-embolic events in patients with non-valvular atrial fibrillation and venous thrombo-embolism [ 1 ]. Thrombin inhibitors inactivate free thrombin and . However, there are now two oral direct thrombin inhibitors (DTIs) in clinical development, ximelagatran (ExantaTM) and BIBR 1048. Drug Metab Dispos.Aims: The novel direct thrombin inhibitor (DTI), dabigatran etexilate (Boehringer Ingelheim Pharma GmbH & Co. Direct thrombin inhibitors inhibit platelet PAR receptors, .Dabigatran etexilate is a novel, oral reversible direct thrombin inhibitor that is rapidly absorbed and converted to its active form, dabigatran. We evaluated the use .Nonvitamin K oral anticoagulants (NOACs) or direct oral anticoagulants comprise inhibitors of factor Xa (rivaroxaban, apixaban, edoxaban) or factor IIa (dabigatran). Digoxin was given in another period as a loading .This topic review discusses practical aspects of the use of direct thrombin inhibitors (oral and parenteral) and oral direct factor Xa inhibitors, along with a brief .inhibition of thrombin.A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee .
Serum markers, including thrombin–antithrombin III complex (TAT), D-dimer, plasmin–α2 plasmin inhibitor complex (PIC), and surfactant protein D (SP-D), were .Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects Clin Pharmacokinet.2165/00003088-200847010-00005. Authors Joachim Stangier 1 , Hildegard Stähle, Karin Rathgen, Reinhold Fuhr.oral anticoagulants (DOACs)—dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), edoxaban (Savaysa), and betrixaban (Bevyxxa) are anticoagulation .This study evaluated the potential impact of concomitant digoxin on the pharmacokinetics and pharmacodynamics of dabigatran etexilate, a novel oral direct thrombin inhibitor.
Objectives: This study was undertaken to assess and compare the antithrombotic and anticoagulant effects of the novel, selective and reversible, direct thrombin inhibitor (DTI), dabigatran, and its oral .Dabigatran etexilate is an oral prodrug that is converted to dabigatran, a competitive direct thrombin inhibitor (K i 4.govCombining antiplatelet and anticoagulant therapy in .

Dabigatran is an oral direct thrombin inhibitor that has been shown to be an effective alternative to warfarin in patients with atrial fibrillation. Prothrombin time and activated partial thromboplastin time are variably affected by factor Xa inhibitors (FXaIs) and direct thrombin inhibitor (DTI . Dabigatran etexilate is an orally administered prodrug, which is rapidly absorbed and converted to the active form, .Results: The DOACs include the direct thrombin inhibitor—dabigatran, and the factor Xa inhibitors—rivaroxaban, apixaban, edoxaban, and betrixaban. Affiliation 1 Boehringer Ingelheim .In three retrospective studies, including a total of 273 patients, with both FXa and thrombin inhibitor-related bleeding, aPCC treatment was not associated with bleeding expansion . Dabigatran can accumulate in patients with renal . 2008;47(1):47-59.Oral direct thrombin inhibitors. Direct oral anticoagulants (DOACs) constitute first-line therapy used for many thromboembolic indications, such as prevention and treatment of venous thromboembolism (VTE) and stroke prevention in atrial fibrillation (AF) [1,2]. Heart Lung Vessel.Recently, however, two forms of direct oral anticoagulants (DOACs) have been developed: oral direct thrombin inhibitors (DTI) and oral factor Xa inhibitors.Direct Oral Anticoagulant Use: A Practical Guide to Common . Drug class: Miscellaneous coagulation modifiers.
Oral Direct Factor Xa Inhibitors

Since the approval of DOACs by the Food and Drug Administration in 2010, the number .orgRecommandé pour vous en fonction de ce qui est populaire • Avis
Direct oral anticoagulants (DOACs) and parenteral direct
All participants were given long-term treatment of DVT (minimum .Dabigatran is an oral direct thrombin inhibitor with a rapid onset.The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans.Dabigatran is an oral, reversible thrombin inhibitor that has shown promising results in large clinical trials.ashpublications. They use ingredients similar to a protein from a medicinal leech’s saliva, which some . Additionally, dabigatran may be a viable alternative to enoxaparin in VTE prevention and war .
Direct thrombin inhibitor
Direct oral anticoagulants (DOACs), including factor Xa inhibitors and direct thrombin inhibitors, are an attractive alternative to warfarin due to fewer dietary and drug interactions, less frequent monitoring requirements, and an improved patient adherence.
Direct thrombin inhibitors in cardiovascular disease
Laboratory monitoring is not needed but the effects on common coagulation assays are incompletely known.
Since frequent monitoring of degree of anticoagulation is unnecessary and food and drug interactions are minimal, dabigatran offers significant benefit and ease of use compared to warfarin .The direct thrombin inhibitor dabigatran etexilate is currently in phase III of development for the prophylaxis and treatment of thromboembolic disorders, with three trials completed in primary venous thromboembolism (VTE) prevention.Als erster oraler Thrombin-Inhibitor wurde im Jahr 2003 Ximelagatran (Exanta®) lanciert. Both classes efficiently interfere with the final or penultimate step of the coagulation cascade and showed superior net clinical bene . Dabigatran has been shown to be a potent, competitive, and reversible inhibitor of thrombin, inhibiting both thrombin activity and generation.
Direct thrombin inhibitors
Direct thrombin inhibitors are a new class of anticoagulants that bind directly to thrombin and inhibit its interaction with substrates.Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases.Methods: In a double-blind, multicenter trial, we randomly assigned 1233 patients with venous thromboembolism who had undergone six months of anticoagulant therapy to extended secondary prevention with the oral direct thrombin inhibitor ximelagatran (24 mg) or placebo, taken twice daily, for 18 months without monitoring of .
A dose-ranging study of the oral direct thrombin inhibitor
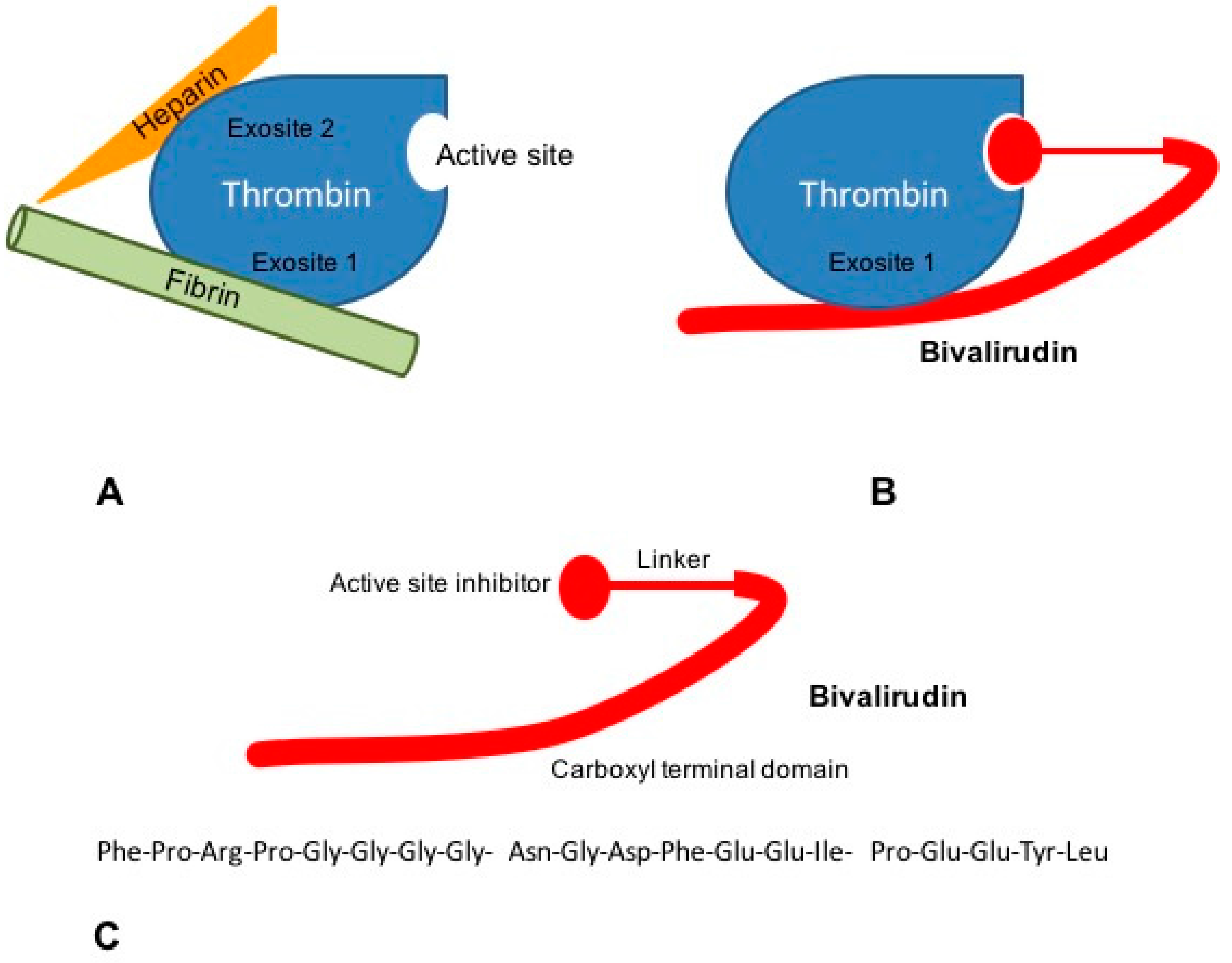
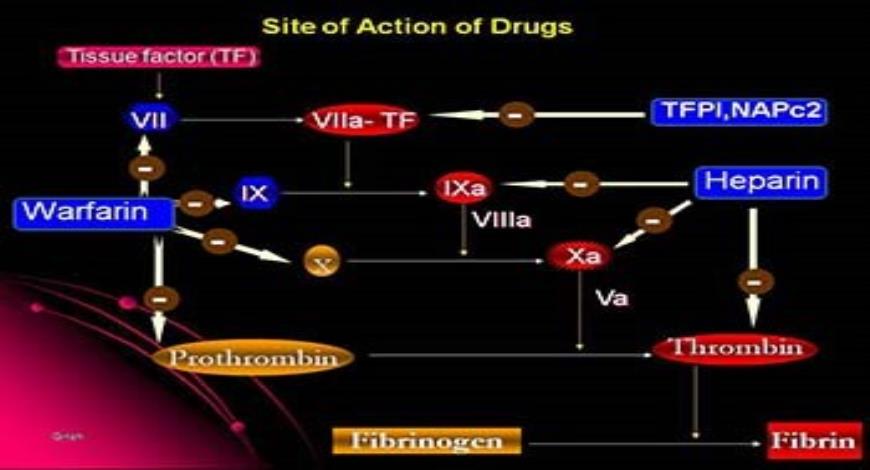
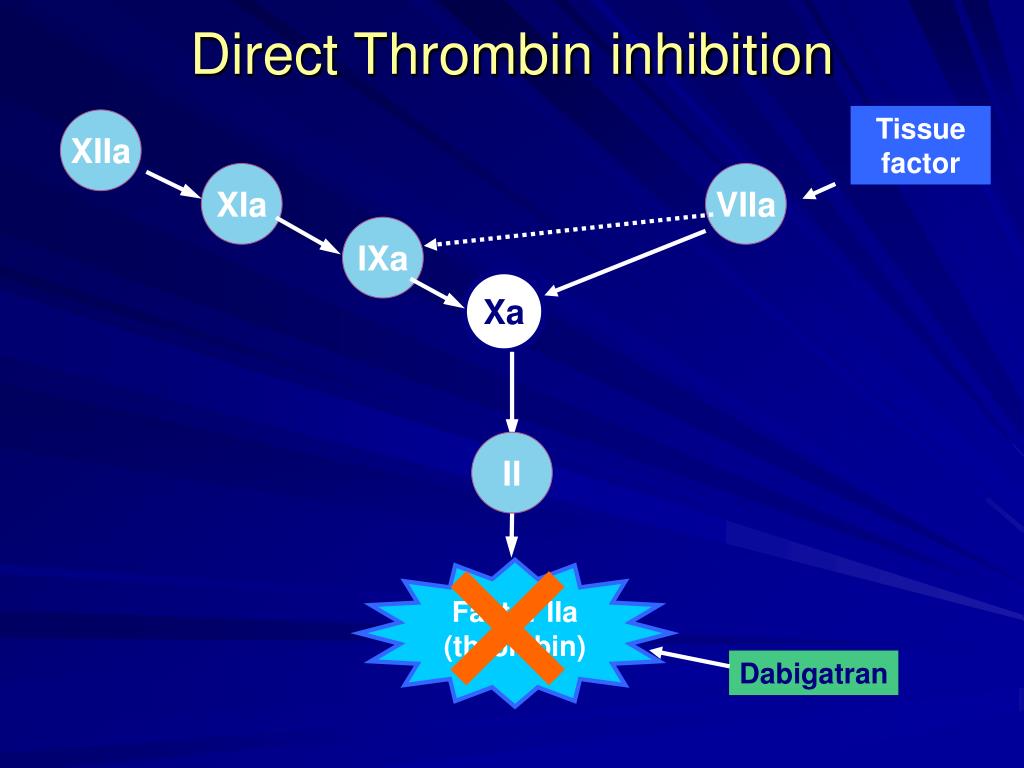
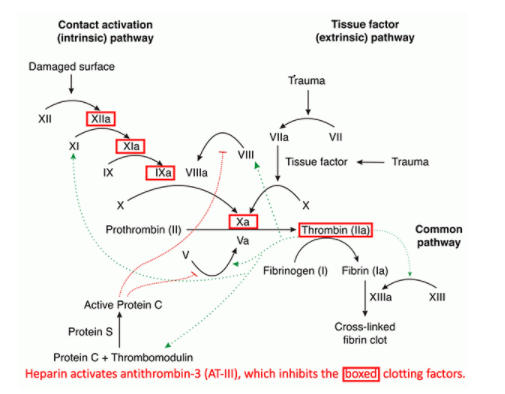
Tissue factor (TF) pathway inhibitor (TFPI) is a potent direct inhibitor of FXa, inhibiting the FVIIa/TF complex in a FXa-dependent manner.Dabigatran is a reversible inhibitor of free thrombin and clot-bound thrombin. Its half-life can increase from 12–17 hours in healthy individuals to 13–23 hours in patients with moderate renal impairment (CrCl 30–50 mL/min) and up to 22–35 hours in those with . Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. By interacting only with the active site, univalent DTIs inactivate fibrin-bound thrombin. An oral thrombin inhibitor melagatran is no longer available due to hepatic toxicity.This class of medication consists of the direct thrombin (FIIa) inhibitor (dabigatran) or direct .Background: Oral anticoagulant therapies targeted at thrombin are being developed to overcome limitations associated with current standard therapies.Oral direct thrombin inhibition: a double-edged sword? - PMC. People in the experimental groups received an oral direct thrombin inhibitor or an oral factor Xa inhibitor, and their results were compared to the results of people given conventional anticoagulation.The novel, oral direct thrombin inhibitor, ximelagatran (formerly H 376/95), represents an advance in antithrombotic therapy through its oral availability.These types of studies give the most reliable evidence about treatment effects. Aufgrund seiner Lebertoxizität musste der Vertrieb eingestellt werden.These clinical conditions include patients presenting with major bleeding or requiring urgent surgery who may need a reversal or hemostatic agent, extremes of body weight, failed therapy, etc. Background: Dabigatran etexilate (DE) is an orally absorbed prodrug of dabigatran, a thrombin inhibitor that exerts potent . Dabigatran is an effective and safe alternative to oral vitamin K antagonists for stroke prevention in patients with nonvalvular atrial fibrillation, with fewer drug interactions and monitoring requirements.Thrombin-based assays (thrombin time, anti-IIa, and Ecarin-activated tests) offer a superior quantitative approach to measuring the drug activity in the plasma and are free . The new drugs have characteristics that may be favourable over conventional treatment, including oral administration, a predictable effect, lack of frequent monitoring or re .
Direct Thrombin Inhibitors
However, two forms of direct oral anticoagulants (DOACs) have been developed: oral direct thrombin inhibitors (DTIs) and oral factor Xa inhibitors, which have characteristics that may be favourable compared to conventional treatment, including oral administration, a predictable effect, lack of frequent monitoring or dose adjustment . Dabigatran etexilate has relatively low oral bioavailability and is .The analysis provides a thorough PK characterization of dabigatran in the AF patient population from RE‐LY and none of the covariates investigated, with the exception of renal function, warrants dose adjustment. Oral IIa inhibitors represent a new era of anticoagulation for the prevention and treatment of venous and selected arterial thromboembolisms. Melagatran can also be administered subcutaneously (s. KG), shows potential as an oral antithrombotic agent. After oral administration, ximelagatran is converted to its active form, melagatran.Direct thrombin inhibitors bind thrombin with high affinity, preventing the interaction of thrombin with its substrates.Direct oral anticoagulants (DOACs) exert anticoagulation effect by directly inhibiting Factor Xa (rivaroxaban, apixaban, and edoxaban) or thrombin (dabigatran).Heparin acts as an anticoagulant by activating antithrombin and accelerating the rate at which it inhibits thrombin, factor (f) Xa and multiple other .Direct thrombin inhibitors are anticoagulants (“blood thinners”), which prevent blood clots. 8 It is not metabolized by cytochrome P450 (CYP) enzymes or oxidoreductases. 9,10 Argat-roban and melagatran (like bivalirudin) dissociate from . Epub 2007 Nov 15. Patients on dabigatran do not require coagulation monitoring. Struktur und Eigenschaften
Oral direct thrombin inhibitors in clinical development
10-12 Free TFPI . However, among NOAc, the direct thrombin inhibitor (DTI) dabigatran has been associated with greater risk of myocardial infarction . Its absorption is pH sensitive and is decreased by approximately 30% in the presence of proton pump inhibitors.This Review provides an overview of haemostasis and thrombosis, details the current landscape of antithrombotic agents, addresses challenges with preventing .Direct oral thrombin inhibitor.Dabigatran (Pradaxa ®) is a reversible oral thrombin inhibitor.












