Ovarian malignancy risk roma

9%) were diagnosed benign on .
Dépistage biologique du cancer de l’ovaire
A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification .
Il permet de classer les patientes selon leur .Diagnostic Performance of Risk of Malignancy Algorithm (ROMA), Risk of Malignancy Index (RMI) and Expert Ultrasound Assessment in a Pelvic Mass Classified as Inconclusive by International Ovarian Tumour Analysis (IOTA) Simple Rules .
Objective: Differentiation between benign and malignant ovarian neoplasms is essential for creating a system for patient referrals.Risk of Ovarian Malignancy Algorithm (ROMA).
Manquant :
The clinical utility of this regression model has been demonstrated in both pre- (75.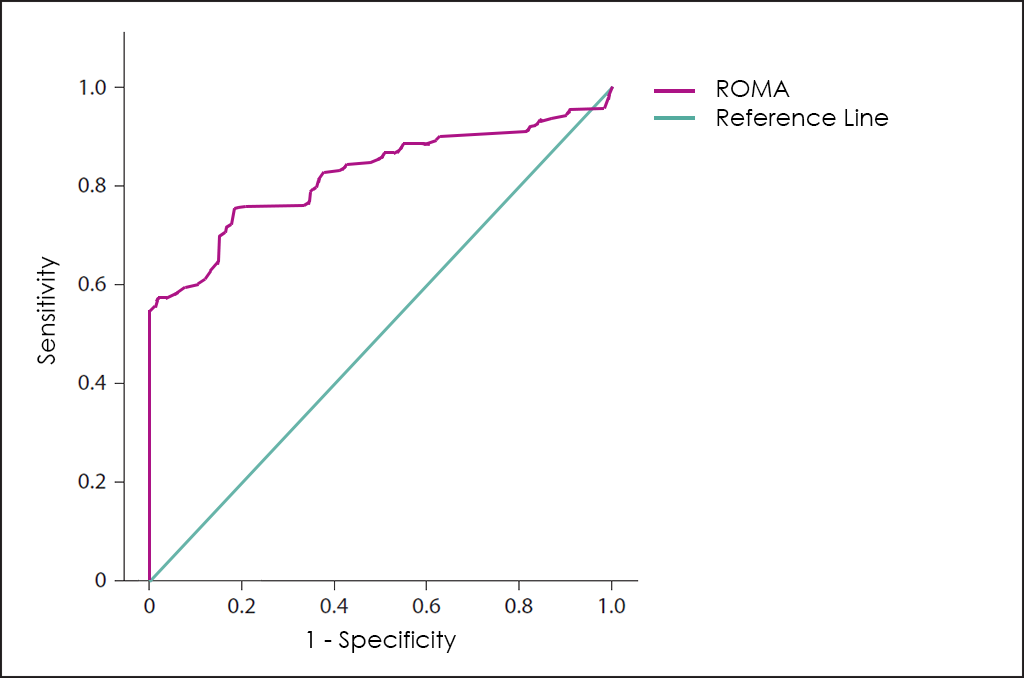
To compare the diagnostic accu . Dikmen ZG, Colak A, Dogan P, Tuncer S, Akbiyik F. There were 94 patients with adnexal masses included in the study, 65 (69.

Objectives: To evaluate the validity and compare the performance of cancer antigen-125 (CA-125), human epididymis protein 4 (HE4), the risk of malignancy index (RMI), and the risk of ovarian malignancy algorithm (ROMA) in the diagnosis of ovarian cancer in patients with ovarian lesions discovered during their preoperative work-up investigations. 2012; 127 (2):379–383.Purpose: To perform a systematic review and meta-analysis of studies comparing the diagnostic accuracy of Risk of Ovarian Malignancy Algorithm (ROMA) and risk of . PMID: 22666786. Food and Drug Administration has approved the Risk of Ovarian Malignancy Algorithm (ROMA) test to assess the presurgical likelihood of ovarian cancer in an adult with an. Multiple studies have shown the effectiveness of ROMA for assessing the risk of malignancy in women with pelvic masses (Table 1). The Risk of Ovarian Malignancy Algorithm (ROMA) combines the results of HE4, CA125, and menopausal status into a numerical score.Karlsen MA, Sandhu N, Høgdall C, et al. ROMA is indicated for women who meet the following criteria: older than . 2016;31:336–44.Conclusions: The ROMA is a simple scoring system which shows excellent diagnostic performance for the detection of EOC in post-menopausal women, but not in pre . developed the Risk of Ovarian Malignancy Algorithm (ROMA) by integrating serum CA-125 and HE4 values and menopausal status to differentiate between low- and high-risk patients with OC [9]. An evaluation of the applicability of . To obtain higher diagnostic efficiency, we created a new diagnostic index, Risk of Ovarian Malignancy Index (ROMI), by combing thymidine kinase 1 (TK1), . The clinical utility of this regression model has .The malignancy algorithm (ROMA) risk utilizes a mathematical formula that incorporates HE-4 and CA 125 levels adjusted for pre and post-menopausal status to determine the risk of malignancy. The Risk of Ovarian Malignancy Algorithm (ROMA ®) is a qualitative serum test that combines the results of HE4, CA125 and menopausal status into a numerical score. ROMA was then subsequently validated in a multicenter trial assessing women that .Ovarian Malignancy Risk-ROMA Test Number 140045 Specimen 1 ml serum in a red-top or gel-barrier tube. Ziauddin University Hospital, Karachi, from May 2014 to June 2015.The medical records of 876 women with ovarian cysts were retrospectively reviewed.3% sensitivity and 74.The clinical value of the ROMA index in the diagnosis of ovarian cancer has been widely reported in the literature, but most of these studies come from small samples .ROMA Intended Use.
Risk of Ovarian Malignancy Algorithm: ROMA®
CA125 and HE4 can also be ordered .In 2009, Moore et al.
For postsurgical monitoring, LabCorp offers the ovarian cancer monitor profile, which includes results for both CA125 and HE4. In 2015, Karlsen et al. The ROMA score considers only the patient’s menopausal status in combination with serum CA125 and HE4 levels and does not incorporate imaging. Study design: Observational study.1%) had epithelial ovarian cancer and 29 (30.In premenopausal women, a risk of ovarian malignancy algorithm (ROMA) value of 1. If the patient is postmenopausal, then a ROMA score . Our study elucidates the diagnostic performance of Risk of Ovarian Malignancy Algorithm (ROMA), Human epididymis secretory protein 4 (HE4) and cancer antigen (CA125). Risk of Ovarian Malignancy Algorithm (ROMA) HE4 assays are recommended for use in conjunction with CA125 . The ROMA is an index based on evaluation of serum CA 125 and HE4 levels in combination with the hormonal status of patients with adnexal tumors.To evaluate the ability of carbohydrate antigen 125 (CA125), human epididymis protein 4 (HE4), risk of ovarian malignancy algorithm (ROMA), and . The Risk of Ovarian Malignancy Algorithm (ROMA) algorithm was approved by the FDA in 2011. The ROMA is a valuable screening test that takes advantage of the high specificity of HE4 and high-sensitivity of CA-125 to detect more . [Google Scholar] 26. developed the Copenhagen Index (CPH-I) based on these two biomarkers and patient .The Risk of Ovarian Malignancy Algorithm (ROMA), which is Food and Drug Administration (FDA) approved, combines CA125, human epididymis protein-4 (HE4) and menopausal status [9, 10] but does not .
140045: Ovarian Malignancy Risk (ROMA®)
Ovarian cancer is not a single disease and can be subdivided into at least five different histological subtypes that have different identifiable risk factors, cells of . Therefore, the contributions of the tumor markers CA125 and human epididymis protein 4 (HE4) as well as the risk ovarian malignancy algorithm (ROMA) and risk malignancy index (RMI) values were considered individually .Test Code ROMA ROMA Score (Ovarian Malignancy Risk Algorithm), Serum.
ROMA Score (Ovarian Malignancy Risk Algorithm), Serum
Reporting Name. Research demonstrates that examining levels of HE4 and CA125 using the ROMA algorithm shows the highest accuracy in determining ovarian cancer risk in pre- and post-menopausal women who have an ovarian mass present. Place and duration of study: Dr.14 is consistent with a low likelihood of finding a malignancy on surgery.8% specificity) and post-menopausal (92.Évolution
Diagnostic measures comparison for ovarian malignancy risk in
vii Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, Skates SJ.
Risk of Ovarian Malignancy Algorithm
Article Google Scholar Chopra S, Vaishya R, Kaur J.14 or greater indicates a high risk of finding epithelial ovarian cancer, whereas a ROMA value . Sensitivity, specificity, and the receiver operating characteristic (ROC) curve analyses of these tumor markers were . Obstet Gynecol . Material and methods: Research was conducted among 456 patients qualified for surgery due to .
Manquant :
Its diagnostic accuracy is variable. Siew Fei Ngu, 1 Yu Ka Chai, 2 Ka Man Choi, 3 Tsin Wah Leung, 4 Justin Li, 5 Gladys S.The risk of ovarian malignancy algorithm (ROMA) incorporates cancer antigen 125 (CA125), human epididymal protein 4 (HE4), and menopausal status to assign women that present .Our study elucidates the diagnostic performance of Risk of Ovarian Malignancy Algorithm (ROMA), Human epididymis secretory protein 4 (HE4) and cancer .Interpretive Data.ROMA — Early Detection Research Network
Auteur : Arpita Suri, Vanamail Perumal, Prajwal Ammalli, Varsha Suryan, Sanjiv Kumar BansalL'algorithme ROMA évalue un risque de malignité, en associant les mesures sériques d'HE4, CA125 et le statut ménopausal.The dual marker algorithm Risk of Ovarian Malignancy Algorithm (ROMA) has been widely used in the clinic for the identification of equivocal pelvic masses in ovarian carcinoma.Score ROMATM (Risk of Ovarian Malignancy Algorithm) Ayant des voies d’expression indépendantes le CA125 et la HE4 peuvent se compléter; l’utilisation de la HE4 permet .Epithelial ovarian cancer has become the most frequent cause of deaths among gynecologic malignancies. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass.PMCID: PMC8833816. 2009;112(1):40-6.0% could be obtained with the suggested cutoff of 7.Risk of Malignancy Algorithm (ROMA), as first described by Moore in 2009, is one of the most popular approaches. If the patient is premenopausal, then a ROMA score of less than 1.The risk of ovarian malignancy algorithm (ROMA) is used for assessing ovarian cancer risk in women with a pelvic mass.

PMID: 35159077.The Risk of Ovarian Malignancy Algorithm (ROMA) was developed from the combination of two pilot studies and employs serum levels of HE4 and CA125 along with menopausal status to stratify patients into high and low risk groups for epithelial ovarian cancer(13;14). The dual-marker algorithm achieved significantly higher sensitivity for identifying women with . Methodology: Two hundred and sixty postmenopausal women of 40-65 years of age with ovarian masses, .
Risk of Ovarian Malignancy Algorithm versus Risk Malignancy
Risk assessment for finding an ovarian malignancy .
ROMA, an algorithm for ovarian cancer
We investigated whether the clinically acceptable minimal sensitivity of >80.Objective: To determine the diagnostic accuracy of ROMA in postmenopausal women with history of ovarian mass.
Ovarian cancer
6% sensitivity and 74.Of the 498 patients assessed, 392 (79%) had benign disease, 22 (4%) had LMP tumors, 28 (6%) had stage I-II epithelial ovarian cancer (EOC), 36 (7%) had stage III-IV EOC and .