Pcl3 lewis structure molecular geometry
It appears as brownish-yellow gas. In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an unshared pair of . It is a monovalent anion and a strong . (c) The actual bond angles deviate slightly from the idealized angles, because the lone pair takes up a larger region of .Oxygen dichloride has the chemical formula OCl 2 with a molar mass of 86. POCl3 Molecular Geometry.
PCl3 Lewis Structure: How to Draw the Dot Structure for PCl3
PCl3 Geometry and Hybridization
Therefore, the electron geometry is tetrahedral while the molecular geometry is trigonal pyramidal. POCl3 Bond Angle.Molecular Geometry: The electron arrangement in compounds is determined by the Lewis structure of the compound. With Hydrogen on the top and Chlorine atoms below, the molecular shape is shown in the figure.edu/jmol/molecules/pcl3.PCl - Phosphorus Trichloride: First draw the Lewis dot structure: Electron geometry: tetrahedral. The O=P-Cl bond angle is 109. It states that valence electrons will assume an electron-pair geometry that minimizes repulsions between areas of high electron density (bonds and/or lone pairs).7: The Shapes of Molecules. Now if we look at the electronic structure in the ground state of phosphorus it will be 1s 2, 2s 2, 2p 6, 3s 2, 3p 2. Here’s the best way to solve it. It states that valence electrons will assume an .
Hybridization of PCl3
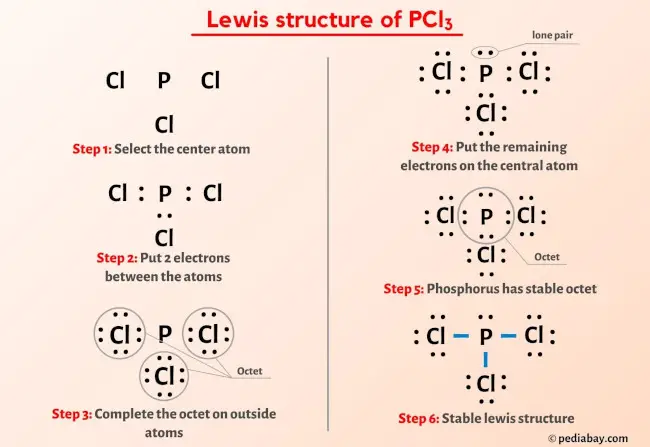
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons.There is a three step approach to determining the geometry of a molecule.
Strategy: Draw the Lewis electron structure of the molecule or polyatomic ion. Now, boron is less electronegative, which makes it the central atom.

The Learning Objectives of this Module: To use the VSEPR model to predict molecular geometries.
Solved ArF2 and PCl3 a) the Lewis structure b) The electron
6K views 10 years ago.comSolved What is the molecular geometry of PCl3? Enter the | .In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom.The shape of a PCl_3 molecule . e) annotate the 3D representation with bond dipoles and molecular dipoles.From the stable Lewis structure above, there are no lone pairs.PCl3 lewis structure has a Phosphorus atom (P) at the center which is surrounded by three Chlorine atoms (Cl).
(b) The trigonal pyramidal molecular structure is determined from the electron-pair geometry. NCl3 is the chemical formula for Nitrogen trichloride. There are two Oxygen atoms bonded to the central Sulfur atom.{ name:jmolApplet0_object,applet:true,documentBase:https://www. Oxygen dichloride is a member of the chlorine oxide family of . Determine the Electron geometry from the .
The valence-shell electron-pair repulsion (VSEPR) model allows us to predict which of the possible structures is actually observed in most cases.If we look at the molecule of PCl3 it is made up of phosphorus and chlorine molecules However, the hybridization basically occurs within the central atom which is phosphorus. The PCl3 Lewis Structure is a diagram that shows the arrangement of electrons in the molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Then draw the 3D molecular structure using VSEPR rules: Click and drag the molecle to rotate it. Using the steric number . Definition and concept. [ClO 3] – is the chemical formula for chlorine trioxide, more commonly known as the chlorate ion.

PCl3 Lewis Structure in 6 Steps (With Images)
5°
PCl3 Lewis structure: Drawing, Hybridisation, Geometry
Lewis electron structures give no information about molecular geometry, the arrangement of bonded atoms in a molecule or polyatomic ion, which is crucial to understanding the chemistry of a molecule. See the Lewis structure of PCl3 and practice problems with solutions. Phosphorus trichloride is made up of one . As per VSEPR theory, the number of bonded (bond pair) and non-bonded (lone pair) valence shell electrons determine the shape and molecular geometry of the molecule.The PCl3 Lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability.PCl 3 lewis structure is made up of three P-Cl bonds, with a phosphorus (P) atom in a central position and all three chlorine (Cl) as outer atoms in the lewis diagram. Now, the polarity: The . It is soluble in water. Decision: The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom.Temps de Lecture Estimé: 1 min
PCl3 Molecular Geometry / Shape and Bond Angles
We can use the A-X-N concept and its table to verify . It is created by representing the valence . The chlorate ion is present in chloric acid salts.To determine the molecular geometry of Chlorine Pentafluoride, we go back to its Lewis structure. It is the simplest form of Sulfur Chloride and exists as a cherry . Let us apply the lewis dot rules and try to draw the structure of boron trichloride.PCl₃ (Phosphorus trichloride) has a trigonal pyramidal Lewis structure: central phosphorus (P) atom with 5 valence electrons forms three single bonds with . To determine the molecular geometry of Sulfur Dioxide, we must observe its Lewis structure.3: Molecular Structure and Polarity.
Predicting Geometry with VSEPR
POCl3 Hybridization.

The total number of valence electrons available for drawing the POCl3 Lewis structure is 32.comRecommandé pour vous en fonction de ce qui est populaire • Avis
5 Easy Steps on PCl3 Lewis Structure, Hybridization (Solved)
BCl3 Lewis Structure.VSEPR to predict Molecular Geometry. Hybridization: sp 3.Once we know the Lewis structure and hybridization of the compound, it becomes easy to understand the molecular geometry of the compound. This is a video worked example for the . A Lewis Structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared electron pairs (bond pairs) or unshared electron pairs (lone pairs).Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.Check me out: http://www. A shared pair of electrons is represented as a short line (a single bond).Question: 13 1 point Molecular Geometry (MG) Look at the Lewis structure for the phosphorous trichloride molecule, PCl3, and determine its molecular geometry. The chemical formula SCl2 represents Sulfur Dichloride. For the PCl3 structure use the periodic table to find the total number of valence. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Assign an AX m E n designation; then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations in bond angles.9° while the I-F bond lengths are 184. In this tutorial, we will learn . Therefore, CHCl3 has a Tetrahedral molecular geometry. Chlorine, group 7, but we have three of those so we have 5 plus . Understanding the molecular . Also, called trichloramine it is a halogen nitride that is yellow and oily with a pungent smell.We have previously discussed the Lewis structures of CO2, O3, SO2, SO3, and more. Chlorine has a steric number of six (five covalent bonds + one lone pair), meaning that there are 12 valence electrons attached.
PCl3 : Lewis Structure and Molecular Geometry
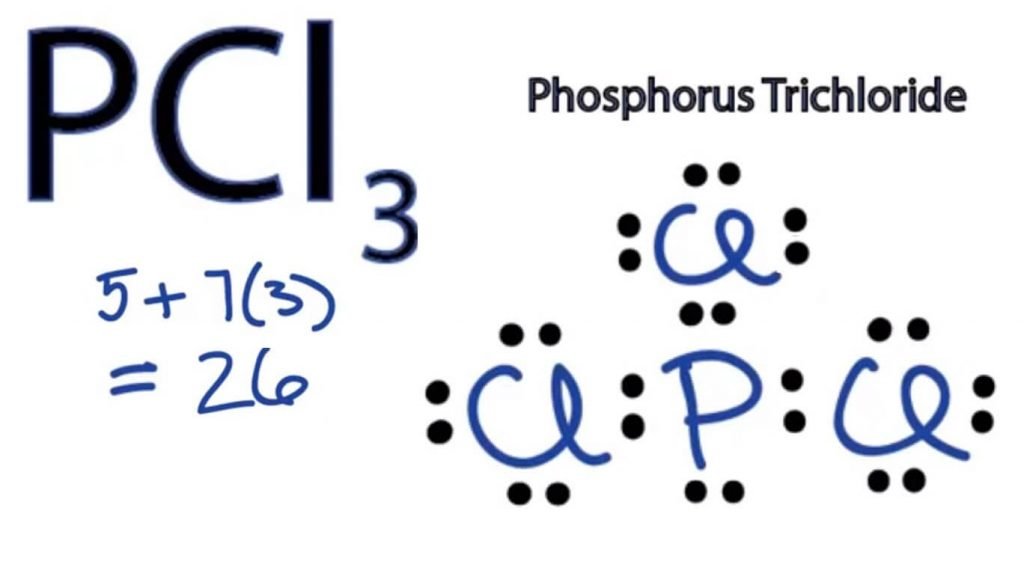
The valence shell in phosphorus is 5. Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape.SiH4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity.
PCl3 Lewis Structure
The molecule of Ozone has three oxygen atoms.

NCl3 Lewis Structure, Geometry, Hybridization, and Polarity. You can follow these four steps to predict the geometry around an atom using VSEPR: Draw the Lewis electron structure of the molecule or polyatomic ion. Determine the Lewis dot structure of the compound. (a) The electron-pair geometry for the ammonia molecule is tetrahedral with one lone pair and three single bonds.February 7, 2020. This is not unusual since chlorine is hypervalent and can possess an expanded octet.8° while the Cl-P-Cl bond angle is 103° in POCl 3.SCl2 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle and Shape.Ozone is one of the most common examples used to study the Lewis structure.Asked for: molecular geometry.Geometry of Molecules. For the central atom of interest, assign the AX m E n designation and the total number of groups ( m + n ). Looking at the PCl3 Lewis structure we can see that there are three atoms.SO2 Molecular Geometry and Shape.The central atom has 3 atoms and a lone pair (SN = 4).Bond Angle : Approximately 109. PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization.Learn how to calculate the molecular and electron geometry of PCl3 using the VSEPR theory, the steric number and the sp3 -hybridization.Chlorate [ClO3]- ion Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non-polar. VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. This indicated a bent molecular shape. SiH4 is the structural composition of silane/silicane.Let's do the Lewis structure for PCl3. The F-I-F bond angle in IF 5 is 81. Here’s a step-by-step . POCl3 Lewis Structure. To understand the . As the central atom has four bonded pairs and sp3 hybridization, the .html,platform:J. It can be observed from the Lewis structure that Iodine, the central atom, has three bond pairs and two lone pairs of electrons. Iodine pentafluoride (IF 5) is a polar molecule with net μ=4.Platform,fullName . There is also a lone pair attached to the Sulfur atom. There are 3 single bonds between the .Lewis Structures.Question: ArF2 and PCl3 a) the Lewis structure b) The electron group geometry c) The molecular geometry d) A 3-D representation e) annotate the 3D representation with bond dipoles and molecular dipoles. POCl3 Valence electrons.The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of .PCl3 : Lewis Structure and Molecular Geometry. Lewis structures show all of the valence electrons in an atom or molecule. It is known as a strong explosive because being unstable in the pure form and sensitive to heat, shock, light, and any organic compound. According to the VSEPR theory, the Chlorine atoms repel each other as much as they can.The molecular geometry or shape of IF 5 is square pyramidal while its ideal electron geometry is octahedral.ICl3 Molecular Geometry.










