Physical properties of europium

Chemical Reaction of Europium.The physical and chemical properties of europium element are mentioned below.Some elements exist in several different structural forms, called allotropes. Structure Determination of Europium Complexes in Solution Using Crystal-Field Splitting of the Narrow f –f Emission Lines. The atomic percentages of europium dopant in In 2 O 3-based solution were y = ([Eu 3 +] [In 3 +]) sol =0; 0.
Europium
Europium (Eu) [63] — Chemical Element — Periodic Table
The transmittance spectra .21 kJ mol -1 Boiling point 1529 °C Enthalpy of vaporization 175. Velocity of sound: (no data) m s ‑1964 and a mass number of 153.Structural, morphological, optical and electrical properties of europium doped In 2 O 3 thin films grown by spray pyrolysis technique are studied in this work. Through this review we explore and assimilate all . 6s2 and the term symbol is 8S7/2. The optical band gap energy decreased with Eu content around 4.Synthesis and Physical Properties of Two Chiral Terpyridyl Europium(III) Complexes with Distinct Crystal Polarity . It has an unusually low density of 5,245 g / cm3, which is significantly lower . The Europium electron configuration is Xe 4f7 6s2. Named for the continent of Europe.The complexes of both europium (III) and terbium (III) are soluble in organic solvents including DMF and DMSO and all the complexes of europium (III) and terbium (III) are stable at room temperature.
It reacts very quickly with water and . How Does Europium Cost.It is a moderately hard, silvery metal which readily oxidizes in air and water.Synthesis and Fluorescence Properties of Divalent Europium -Poly(Methacrylate Containing [2. Chemical Properties of Europium. Materials and instrumentation. Published: 05 October 2022.6 eV below the Fermi level. As Eu 2+ the metal can replace calcium and strontium in certain minerals, such as the calcium feldspars (calcium .Physical properties of europium. For EuTe 4, the density of states stretches to the vicinity of the Fermi level.Enhancing the optical emission of cerium oxide nanoparticles is essential for potential biomedical applications.25 g/cm 3 and its atomic mass is 151.Get the facts about element Europium (Eu) [63] from the periodic table.Europium, basic physical and chemical properties of the element. It is the most active element among the lanthanides.2] Cryptand) Complexes. It belongs to the lanthanide series of the periodic table, which is characterized by the filling of the 4f electron shell.Additionally, we further investigated the physical properties of the two materials. The density of europium is 5.
Europium Price, Occurrence, Extraction and Use
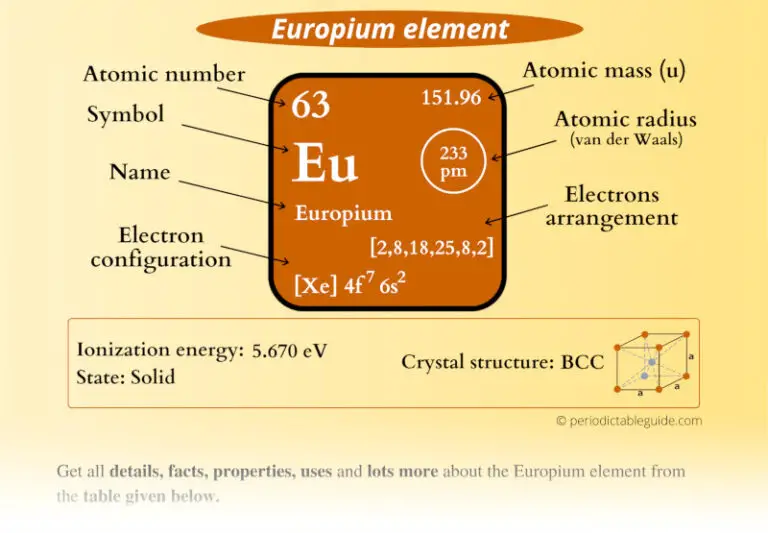
Europium has sixty-three protons and ninety neutrons in its nucleus, and sixty-three electrons in six shells. The melting point of europium is . The Kossel shell structure of europium. In the present work, we report a simple chemical precipitation technique to synthesize europium-doped cerium oxide nanostructures to enhance the emission properties. Europium is obtained from monazite sand, which is a mixture of phosphates of calcium, thorium, cerium and most other rare earths. Uses of Europium. Applications of Europium. The data are compared . Its melting point is 826 O C which is the second lowest melting point.Electrical properties. It has a rock-salt structure, is a deep red solid, and is ferromagnetic at 77 K.Europium is a chemical element with the symbol Eu and atomic number 63. Europium is a soft, ductile metal which is silvery in appearance. It has thirty isotopes with mass numbers ranging .
Europium: Properties and Applications
The unique physical and chemical properties of the RE sesquioxides such as the dense Kondo effect, heavy fermionic behavior, and high dielectric constants, just to mention a .It is paramagnetic above 90 K but is antiferromagnetic below 90 K. State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing 210093, P.Corpus ID: 123659112; On the unusual physical properties of europium-based molybdenum chalcogenides and related Chevrel compounds @article{Meul1986OnTU, title={On the unusual physical properties of europium-based molybdenum chalcogenides and related Chevrel compounds}, author={H.964 (1) Europium was discovered by Eugène-Anatole Demarçay (FR) in 1896. Urbach energy was of the order of 278 meV, it decreased with Eu . Belonging to the family of lanthanides, europium (pronounced as yoo-RO-pee-em) is a rare earth metal denoted by the chemical symbol Eu [1]. The XPS spectra near the Fermi level indicated that EuTe is a semiconductor, with its valence band top located about 0. Belonging to the family of lanthanides, europium (pronounced as yoo-RO-pee-em) is a rare earth metal denoted by . The molar conductance values in DMF lie in the range of 8–11 Ω −1 cm 2 mol −1 indicating non-electrolytic behavior of complexes. Melting point 822 °C Enthalpy of fusion (molar) 9. Meul}, journal={Helvetica Physica Acta}, .Properties of Europium. Thermal properties. Below is a detailed overview of Europium .In2O3 : Eu thin films were successfully grown by spray pyrolysis. It is flexible in nature, its hardness is similar with the lead. Schematic electronic configuration of europium. Atomic weight of Europium is 151.EuropiumTrendsBoronOsmiumNitrogenZinc
Europium
Although europium is present in most of the minerals containing the other rare elements, due to the difficulties in separating the elements it was not until the late 1800s that the element was isolated.B: classification: lanthanides: discovery year: . The physical and chemical properties of europium are also discussed, as well. It is located in group Lanthanides, period six and block f of the periodic . What is Europium.A novel mesoporous SBA-15 type of hybrid material (phen−SBA-15) covalently bonded with 1,10-phenanthroline (phen) ligand was synthesized by co-condensation of tetraethoxysilane (TEOS) and the chelate ligand 5-[N,N-bis-3-(triethoxysilyl)propyl]ureyl-1,10-phenanthroline (phen-Si) in the presence of Pluronic P123 surfactant as a template. An official website of the United States government .Eu: properties of free atoms.The photoluminescence properties of europium (III) and terbium (III) complexes have also been studied. Department of Applied Chemistry, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. The atomic mass of europium is 167. Its boiling point is 1529 O C. It belongs to group null of the periodic table having trivial name . There are many isotopes of europium, and out of those isotopes .

Europium atoms have 63 electrons and the shell structure is 2. You can also download .The properties reported are density, specific heat, thermal diffusivity and conductivity, thermal expansivity, Young's modulus, and strength. The complexes of Europium (III) and Terbium (III) show very strong characteristic emission in red and green regions, respectively.Photochemical Properties of Europium (III) Methylbenzoates. William Crookes observed the phosphorescent spectra of the rare elements including those eventually assigned to europium. The Europium symbol is ‘Eu’.Physical and chemical properties of Europium: general data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information. Chemistry Letters 1993 , 22 (9) , 1555-1558. XRD studies showed that the films had In2O3 cubic structure with (004) preferential orientation and best crystal properties at 1. Electrical resistivity: 90 × 10 ‑8 Ω m; or mΩ cm; Heat and conduction. The atomic structure of Europium is key to understanding its unique properties, especially its luminescence. It also has a high melting point of 1512F (822C) and high boiling point of 2784F (1529C .Physical Properties. The melting point and boiling point of europium is 1099 K and 1802 K respectively. Experimental 2. The reagent and solvents used were of analytical . Here ‘Xe’ stands for Xenon. On this concept page, you will find a proper explanation of the structural, physical, and chemical properties of this element. PHOTOCHEMISTRY AND MAGNETOCHEMISTRY.Chalcogenides Oxides. The YAG crystal, known for its properties . Read more on Wikipedia. It is also electropositive, and its electronegativity on the Pauling scale is 1. Europium was first found in .Physical Characteristics. Reflectivity: (no data) % Refractive index: (no data) (no units) Acoustic properties. Europium is a metal having a silvery white appearance with a pale yellow tint.Physical properties - Europium has the characteristic soft texture and shiny silver appearance of metals.

This lesson is about the discovery of the chemical element Europium.
Europium
Dong-Ping Li, Dong-Ping Li.Physical Insights into Materials and Molecular Properties NEXT.Relative atomic mass: 151.Interesting Facts. Physical properties.
Europium Element
7 kJ mol -1 Density 5. Structural and optical properties showed an acute dependence on . It has low density at room .Europium is one of the more abundant lanthanoids and is almost twice as abundant as tin.Europium: English name: Europium: chemical symbol: Eu: atomic number: 63: relative atomic mass: 151,965: period: 6: group: III.The geometries, stabilities, and electronic and magnetic properties of europium encapsulated EuSi(n) (n=1-13) clusters have been investigated systematically by using relativistic density functional theory with generalized gradient approximation and it is concluded that most of the 4f electrons of the Eu atom in the EUSi(12) cluster do not .
Europium
Its atomic number is 63.Europium is a very strong paramagnet above about 90 K (−183 °C, or −298 °F); below that temperature the metal orders . Periodic Table of the Elements; Europium: Metal: Symbol: Eu Atomic number: 63 Atomic mass: 151.
Facts About Europium
It is the most reactive of the lanthanide group: it tarnishes quickly in air at room temperature, burns at about 150 C to .Physical properties of europium Like the other lanthanides, europium is a silvery, soft heavy metal. There are several uses for europium in the . It has the potential to become a magnetic refrigeration material (ΔS mag =−143 mg/cm 3 K,50 kOe).Europium is a soft silvery metal, both are and expensive. Atomic properties.Physical Properties of Europium.Europium | Eu | CID 23981 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.Europium is the 63rd element in the periodic table and has a symbol of Eu and atomic number of 63. Health Effects of Europium.013 Corpus ID: 99588657; Study on the physical properties of europium doped indium oxide thin films @article{Beji2016StudyOT, title={Study on the physical properties of europium doped indium oxide thin films}, author={Nasreddine Beji and Mehdi Souli and Meriem Reghima and Sonia Azzaza and Safia Alleg and Najoua .Material and Physical Properties. Europium is moderately hard and silvery metal.









