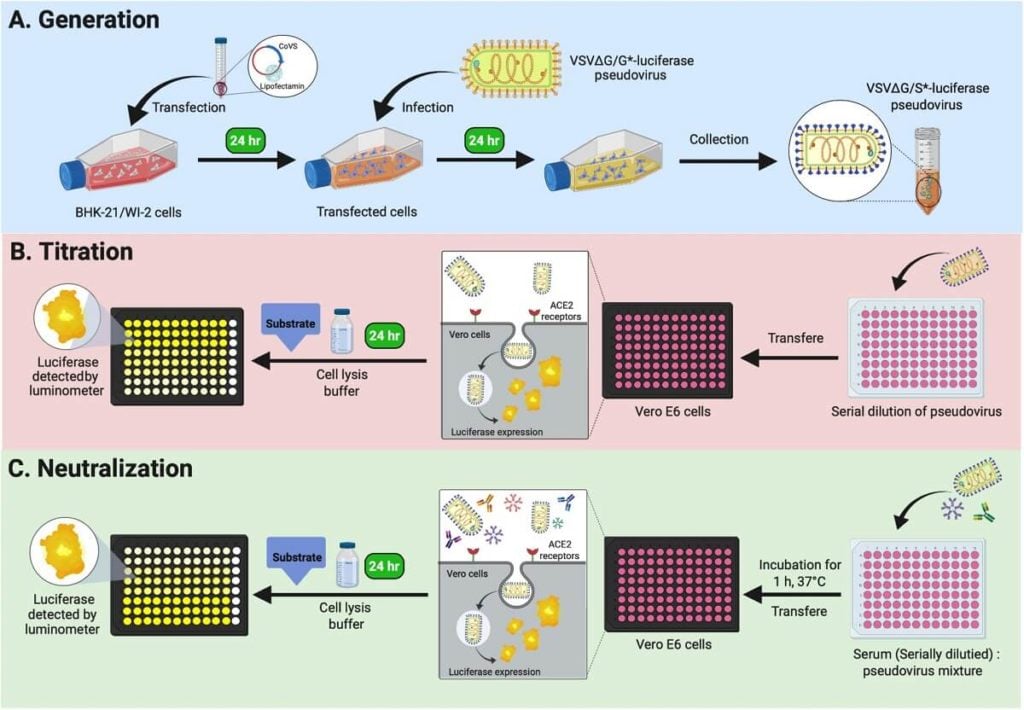
Plaque reduction neutralization test
2016:1426:273-82.The plaque reduction neutralizing test (PRNT) using live viruses is currently the 'gold standard' for the differential serodiagnosis of flaviviruses.PRNT Plaque reduction neutralization test RNA Ribonucleic acid WN West nile WNV West nile virus Abbreviations and acronyms.Since their first documentation in 1952, plaque reduction neutralization tests (PRNTs) have become the choice of test for the measurement of neutralizing antibodies against a particular virus.Background: The plaque reduction neutralization test (PRNT) remains the gold standard for the detection of serologic immune responses to dengue virus (DENV). PRNTs can also confirm acute infection by demonstrating a fourfold or greater change in WNV-specific neutralizing antibody titer between acute- and .3390/v14071560
Advances in Neutralization Assays for SARS‐CoV‐2
Plaque reduction neutralization tests are the “gold-standard” for assessing neutralizing antibody titers with 50% reduction of plaque numbers (PRNT 50) being an .Here we describe a conventional plaque reduction neutralization test (PRNT) to check the presence of antibodies against SARS-CoV-2 in patient samples (serum or plasma).Guidelines for plaque reduction neutralization testing of . Although the surrogate neutralization test exhibits correlation to a plaque reduction neutralization test, the clinical or public health applicability has not been .Auteur : Kevin R Bewley, Naomi S Coombes, Luc Gagnon, Lorna McInroy, Natalie Baker, Imam Shaik, Julien R St-J.Plaque reduction neutralization assays are considered the gold standard for detection of neutralizing antibodies, but require cells, infectious virus, and are difficult to standardize.La méthode choisie pour neutraliser le produit chimique en question doit à tout prix être d'abord testée à petite échelle afin de détecter d'éventuels problèmes imprévus.Virus neutralization assays measure neutralizing antibodies in serum and plasma, and the plaque reduction neutralization test (PRNT) is considered the gold . The BHK semimicro PRNT compared favorably in terms of sensitivity in detecting dengue antibody (96%), specificity at a screening dilution .Neutralization assays are often used as part of research and diagnostics to detect neutralizing antibodies and to determine a possible protective antibody titer after .To investigate neutralizing characteristics, various methods for detection and quantification of antibodies reactive against viral proteins like hemagglutination inhibition .Comparison of plaque reduction and focus reduction neutralization tests for the measurement of neutralizing antibody titers against japanese encephalitis virus - .The gold standard to assess antibody titer is the plaque reduction neutralization test, an end-point assay which evaluates the highest serum antibody .Overview
Plaque Reduction Neutralization Test
Incubate overnight at 4°C However, it is time-consuming, and results can be subjective (owing to . PMID: 38248380.
Plaque Reduction Neutralization Testing (PRNT) has long been considered the standard for viral assays, but other assays can be used to measured cell viability or cell death such as MTT, MTS or Trypan Blue (see sample protocols below).Dengue virus (DENV) infection is a worsening global health problem.Abbreviation: PRNT, plaque reduction neutralization test.All the recombinant viruses were analyzed by means of 50% plaque reduction neutralization testing with 20 human serum samples, collected from 15 .
Neutralization Assay Protocol
L'échantillon de sérum, ou la solution .
Diseases
Employing the criterion of 4-fold greater titer, 78 (10.Neutralization Assay for Chikungunya Virus Infection: Plaque Reduction Neutralization Test Methods Mol Biol.

Plaque reduction neutralizing reference test (PRNT) Samples were assayed twice in parallel in two-fold dilutions ranging from 1:10 to 1:80 (final) in the plaque reduction neutralization test. Neutralization renders the . The most stringent PRNT, which is represented by the 90% endpoint PRNT (PRNT 90 ), is considered the gold standard protocol for the serodiagnosis of flavivirus infections. Assessing NAb levels and understanding the kinetics of NAb responses is critical for evaluating immune protection.Live virus neutralization assays mainly include focus-reduction neutralization test (FRNT), 81 plaque reduction neutralization test (PRNT) 82 and live virus micro-neutralization (MN) assay. The 94 clinical cases of CZS submitted to the ZIKV PRNT 90 test were followed from 2016 to 2018. Presence of nAb inhibits viral entry and the formation of plaques.The classical plaque reduction neutralization test (PRNT) is performed in a 24‐well format in duplicate for each serum dilution. When nAb are absent, spike proteins of SARS-CoV-2 interact with ACE2 receptors (ACE2) to allow viral entry, replication, and subsequent plaque formation.The Plaque Reduction Neutralization Assay (or PRNT) is considered the gold standard to measure antibody neutralization. In PRNT, sera containing the antibodies of interest are serially diluted and incubated with the target virus to form immune complexes. Despite the importance of . Elle est couramment . Plaque Reduction Neutralization Test (PRNT) Accuracy in Evaluating Humoral Immune Response to SARS-CoV-2. Experimental Specifications.intA Plaque Reduction Neutralization Test for the Detection .10 4 PS cells counted with Malassez cells were added to each well of the plate. Figures (0) & Videos (0) Fig.
Neutralisation — Wikipédia
A newly modified semimicro plaque reduction neutralization test (PRNT) in BHK cells was compared with a standard PRNT in bottles with LLC-MK2 monolayers and with an LLC-MK2 PRNT adapted to semimicro methods.The ZIKV plaque reduction neutralization test (PRNT) is the confirmatory serological assay, which detects only the subset of antibodies capable of blocking the interaction of ZIKV with its host cell receptor, effectively inhibiting replication. Add 400 µL of virion suspension, and then add 300 µL of culture medium to each antibody dilution. The Median Lethal Dose (LD50) or neutralizing titer can be calculated by any suitable method. The virus‐serum‐cell mixture is left .2%) for WNV, two (0.

Background The plaque reduction neutralization test (PRNT) is currently the best and most widely accepted approach to measuring virus-neutralizing and protective antibodies to dengue virus, and in assessing the immunogenicity of a dengue vaccine.

Here, we report on a fluorescence-based SARS-CoV-2 neutralization assay that detects SARS-CoV-2 neutralizing antibodies in COVID-19 patient specimens and . Virus Neutralization Assay Neutralization Assay Cell And Tissue . The neutralizing antibody titer was recorded as the reciprocal of the highest serum dilution reducing by at least 80% the number of cell clusters infected by . However, the correlation between presence of dengue-neutralizing antibody and . Many variations of the PRNT are currently in .Le test de séroneutralisation par réduction des plages de lyse permet de quantifier le titre d' anticorps neutralisants contre un virus .The plaque reduction neutralization test (PRNT) is considered the gold standard for measuring neutralization antibodies against SARS-CoV-2 [ 10, 11, 12 ]. The plaque reduction neutralization test (PRNT) is currently considered to be the gold standard to characterize and quantify circulating levels of anti-DENV neutralizing antibody (NAb). The presence of antibody is believed to be most relevant .Plaque Reduction Neutralization Test (PRNT), Applied to Dengue Virus Day 1. The highest serum dilutions neutralizing ≥50% or ≥90% of plaques were regarded as the PRNT 50 and PRNT 90 .La neutralisation ou neutralisation acidobasique est une réaction chimique où un acide réagit avec une base de façon à former de l' eau et un sel 1, 2.
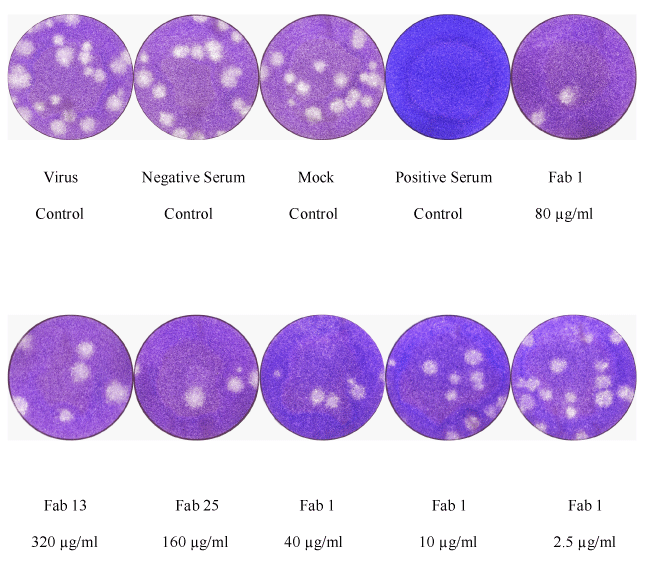
Dilute twofold the antibody and the negative control in culture medium, to obtain two series of five dilutions of 400 µL each.6 in our live virus assay corresponded to the threshold for .Plaque-reduction neutralization test (PRNT) is the current gold standard for detection of nAb.
A high-throughput neutralizing antibody assay for COVID-19
This study analyzes the positivity of the plaque reduction neutralization test (PRNT) in children with clinical or imaging characteristics of CZS and its association with laboratory, clinical, and imaging characteristics.The SONIA neutralizing antibody assay using finger-prick dried blood spots displays 91–97% sensitivity and 100% specificity in comparison to the live-virus . Virus neutralization assays measure neutralizing antibodies in serum and plasma, and the plaque reduction neutralization test (PRNT) is considered the gold standard .
Manquant :
plaque reductionPlaque reduction neutralization test
83, 84 These assays can be used to detect antibodies or screen antiviral drugs that inhibit SARS-CoV-2 replication in vitro.
Diagnostic Testing
After an incubation period of 3–4 h at 37°C, an overlay medium .The reference standard for the quantification of antibodies capable of neutralizing SARS-CoV-2 is the plaque-reduction neutralization test (PRNT).A plaque reduction neutralization test (PRNT) is a method to measure neutralizing antibodies against viruses, such as mumps, Zika, and dengue.The plaque reduction neutralization test (PRNT) is the most specific serological test for the proper serological identification of flaviviruses (4, 6, 7). Techniques Reagents Other Keywords. ThePRNT is the most common assay used to measure neutralizing antibody. The plaque assay is the most widely used method for detecting infectious virions and involves counting discrete plaques in cells.In an attempt to make interlaboratory information more directly comparable, WHO with the support of PDVI initiated a program to coordinate the procedures used for the plaque-reduction neutralization test (PRNT).3%) for Cacipacore . However, PRNTs can be performed only against viruses that cause cytopathic effects (CPE). Briefly, serial dilutions of the patient .PMCID: PMC10814169.
Interim Guidelines for COVID-19 Antibody Testing
3%) equines were seropositive for Ilheus virus, 59 (7.The sera were initially screened by using a blocking ELISA and then titrated by 90% plaque-reduction neutralization test (PRNT90) for 12 flaviviruses.We previously established that a 50% plaque reduction neutralization antibody titer (PRNT 50) ≥25. Neutralizing antibodies (nAbs) against ZIKV are considered a more specific marker to confirm . It involves infecting .

Thereafter, the mixtures were incubated at 37°C for 1 h to allow neutralization of the virus by specific antibodies contained in the diluted serum samples, and 6.