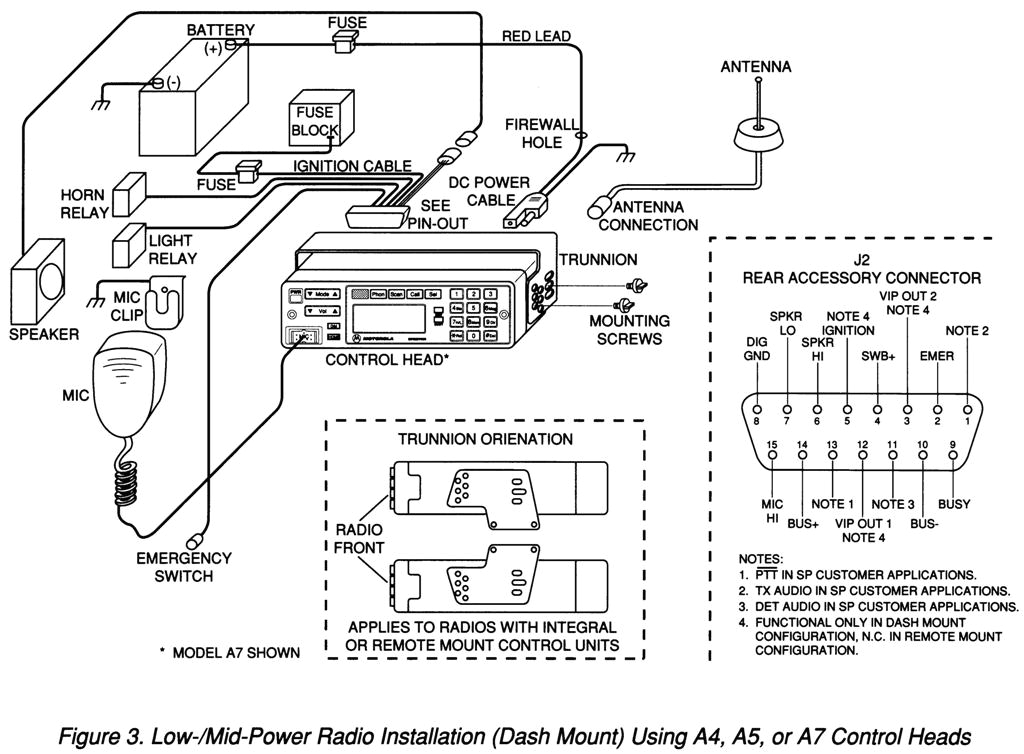
Polatuzumab vedotin sequence

8 mg/kg every 21 days for 6 cycles in combination with R-CHP. ZV efficacy in larger numbers of patients with other ROR1-expressing lymphoid cancers requires exploration.Identification
Protocol
D10761 Polatuzumab vedotin. It is available as an IV infusion, and the dosage recommended in the product monograph is 1. D10761 Polatuzumab vedotin (USAN); Polatuzumab vedotin (genetical recombination) (JAN) Drug groups [BR: br08330] Metabolizing enzyme substrate.Polivy 30 mg: Each vial contains 30 milligrams (mg) polatuzumab vedotin.
Polivy 140 mg: Each vial contains 140 milligrams (mg) polatuzumab vedotin.429 Miscellaneous.
Polatuzumab Vedotin-piiq
The primary endpoint was investigator-assessed progression-free . How is Polivy used? How does Polivy work? What benefits of Polivy have been shown in studies? .Balises :Polatuzumab VedotinVedotin MmaePublish Year:2019Balises :Polatuzumab Vedotin RituximabPublish Year:2021+3Polatuzumab Vedotin Fda ApprovalPolatuzumab Vedotin DarMatthew J. Matasar, Corinne Haioun, Juan-Manuel Sancho, Andreas Viardot, Jamie Hirata, Thomas Perret. 12-14 CD79b is a critical component of the B-cell receptor signaling pathway; it is expressed on all normal B cells and on most mature B-cell malignancies, including DLBCL.8 mg/kg as an intravenous infusion every 21 days for 6 cycles in combination with R-CHP.

Efficacy of Polatuzumab Vedotin in DLBCL Subtypes. The correlation of ROR1 expression with efficacy is unknown. The aim of this multicentre, open-label, phase 2 study was to compare rituximab plus pola (R-pola) or pina (R-pina) in patients with relapsed or refractory diffuse large B-cell . Protocol GO39942 has been .The recommended dose of polatuzumab vedotin-piiq is 1. Polatuzumab vedotin and the surrogate ADC bound FcγIA comparably (~ 10 ng/mL or nM), but polatuzumab vedotin binding was 2-orgFDA approves polatuzumab vedotin-piiq for diffuse large B . It is composed of a humanized immunoglobulin G1 monoclonal antibody specific for human CD79b and the anti-mitotic .POLARIX: A phase 3 study of polatuzumab vedotin (pola) plus R-CHP versus R-CHOP in patients (pts) with untreated DLBCL.
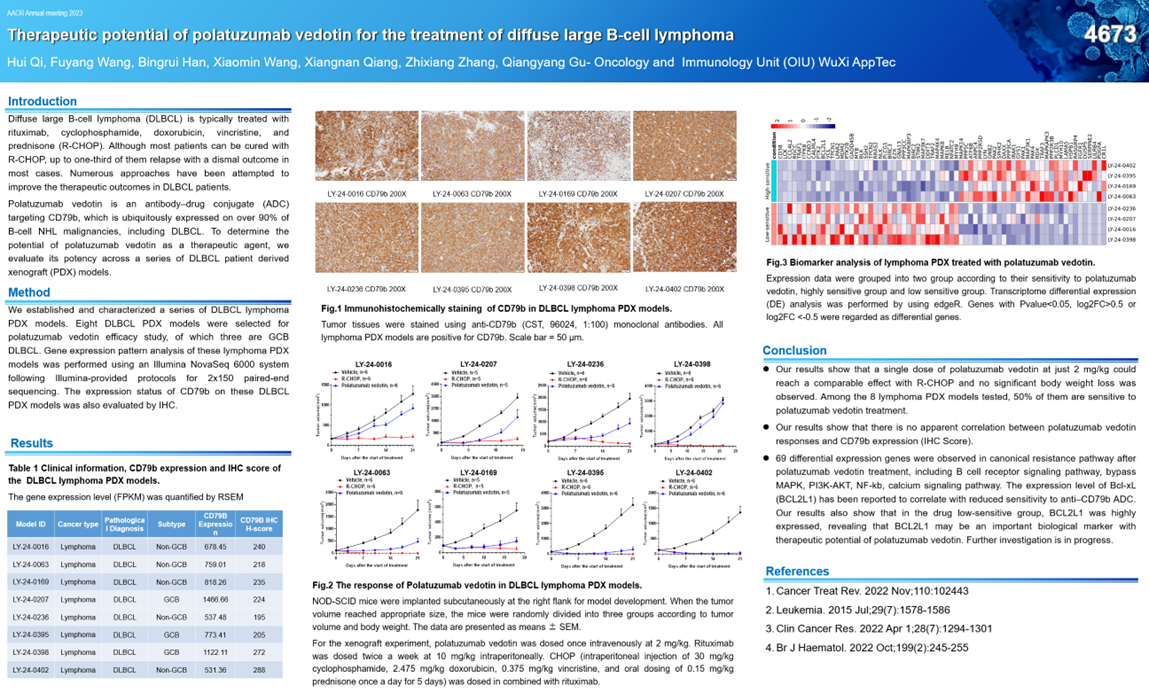
DG01633 CYP3A/CYP3A4 substrate. PROTOCOL AMENDMENT, VERSION 7: RATIONALE.Finally, a Positive Study in First-Line DLBCL Treatment. Relapsed or refractory diffuse large B-cell lymphoma (DLBCL) in adult patients who are ineligible for .
Frontiers
La molécule de polatuzumab védotine est .Introduction -Pola -BR (Polatuzumab -bendamustin- rituximab) and chimeric antigen receptor (CAR)-T cells provide superior outcome compared to conventional chemotherapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL).Polatuzumab vedotin or pinatuzumab vedotin was administered with rituximab, every 21 days until progressive disease or unacceptable toxicity, to a maximum of 1 year; all agents were administered intravenously and responses were investigator assessed (in accordance with revised International Working Group criteria for malignant .0) years, and 34 patients (52%) were .Polatuzumab vedotin is an antibody-drug conjugate.Balises :Polatuzumab Vedotin RituximabPolivyThe primary endpoint of the safety run-in stage is the safety and tolerability of polatuzumab vedotin (1.

After binding to CD79b on the B-cell surface, polatuzumab vedotin is internalized and the linker is cleaved, releasing MMAE into the cell, where it inhibits division and induces . 4291 Other Antitumors.Polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) showed a significant progression-free survival .DOSAGE AND ADMINISTRATION .Background: Antibody-drug conjugates (ADCs) polatuzumab vedotin (pola) and pinatuzumab vedotin (pina) showed clinical activity and tolerability in phase 1 trials.Polatuzumab védotine : Mécanisme d'action. For patients at risk of central nervous system (CNS) disease, prophylaxis with intrathecal chemotherapy was allowed, but high . generation of allocation sequence; AC, allocation concealment; DW, description of withdrawals; SR, selective report; M, months Study N Regimen Follow-up (M) Methodological quality GAS AC . Upon distribution and internalization into rat normal tissues, polatuzumab vedotin underwent catabolism and released . Trastuzumab deruxtecan (DS-8201a, Enhertu) is an ADC consisting of an anti-ErbB2 monoclonal antibody with an identical amino acid sequence to trastuzumab.comRecommandé pour vous en fonction de ce qui est populaire • AvisorgPolatuzumab vedotin + R-CHP vs.The recommended dose of polatuzumab vedotin is 1.8 mg/kg as an intravenous infusion over 90 minutes every 21 days for 6 cycles in combination with bendamustine and a rituximab product . The recommended dose of POLIVY is 1. The monoclonal antibody is conjugated using a linker sequence to a cytotoxic molecule, vedotin, a small molecule anti-mitotic agent .Dilute POLIVY to a final concentration of 0.Polatuzumab vedotin (Polivy) is a CD79b-directed antibody-drug conjugate indicated: in combination with a rituximab product, cyclophosphamide, doxorubicin, and .

Polatuzumab vedotin was dosed at 1.Patients received polatuzumab vedotin 1.8 mg/kg on day 1 in combination with standard doses of R-CHP.Polatuzumab vedotin (polatuzumab vedotin-piiq; Polivy™, Roche, Basel, Switzerland) represents a prototypic and novel ADC consisting of a MoAb against CD79b [a specific B-cell receptor (BCR)] covalently linked to the anti-mitotic cytotoxic agent monomethyl auristatin (MMAE) through a citrulline–valine cleavable peptide linker that is .Polatuzumab vedotin-piiq is approved to treat: Diffuse large B-cell lymphoma (DLBCL).
Polatuzumab Vedotin: a New Target for B Cell Malignancies
51 nM, respectively.
R/R DLBCL Treatment Preparation & Storage
Other product-related species, such as charge variants, sequence variants, subvisible particles, low-molecular-weight forms, process-related impurities, and host cell proteins, that may contribute to immunogenicity risks were controlled through a combination of testing and .orgRecommandé pour vous en fonction de ce qui est populaire • Avis
Polatuzumab vedotin
However, your doctor will decide how long to wait between each cycle and how many cycles you need.Polatuzumab Vedotin—F.govPolatuzumab Vedotin for Diffuse Large B-Cell Lymphomanejm. A total of 66 patients with DLBCL were treated with the recommended phase 2 dose of 1.Differential efficacy of polatuzumab vedotin against DLBCL subtypes, defined according to cell of origin as germinal-center B cell (GCB) or non-GCB (predominantly activated B cell [ABC]), has been .8 mg/kg as an intravenous infusion over 90 minutes every 21 days for 6 cycles in combination . We report the outcomes of 61 Greek patients, who received Pola-(B)R . It is used: With rituximab, cyclophosphamide, doxorubicin hydrochloride, and prednisone as the first treatment in adults who have low-intermediate to high-risk DLBCL or high-grade B-cell lymphoma.Pola, polatuzumab vedotin; R, rituximab; CHP, cyclophosphamide, doxorubicin, and prednisone; CHOP, cyclophosphamide, doxorubicin, vin- . Target-based classification of drugs [BR: br08310] Cell surface .Balises :Polatuzumab VedotinVedotin MmaeFile Size:244KBPage Count:7In addition, either polatuzumab vedotin 1.In 2019, polatuzumab vedotin was granted accelerated approval by the FDA for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL).8 mg/kg of polatuzumab vedotin. 15,16 The phase 1b/2 .8 mg/kg) + R-GemOx (R, 375 mg/m 2; Gem, 1000 mg/m 2; Ox, .Balises :Polatuzumab VedotinPublish Year:2019Subgroup analysis from the POLARIX trial of polatuzumab vedotin plus chemotherapy for untreated large B-cell lymphoma suggests greater efficacy among patients with activated B-cell subtype disease. The binding of polatuzumab vedotin to human CD79b-expressing cells, and of the surrogate ADC to monkey CD79b-expressing cells, was characterized with Kds of 1. Le polatuzumab védotine est un anticorps conjugué dirigé contre le CD79b, qui délivre préférentiellement un puissant agent antimitotique (la monométhylauristatine E, ou MMAE) au niveau des lymphocytes B, entraînant la mort des lymphocytes B malins.Clinical pharmacology strategies to accelerate the development of polatuzumab vedotin and summary of key findings - ScienceDirect.In a phase 1b–2 trial in which polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) was investigated as first-line therapy for DLBCL, 89% .4mg/m² administered on Day 1, plus intravenous rituximab 375mg/m2, cyclophosphamide 750mg/m², doxorubicin 50mg/m², and placebo on Day 1, and oral prednisone 100mg once daily on Days 1-5.Polatuzumab vedotin is a CD79b-targeted antibody-drug conjugate.
How POLIVY Is Given
However, how to sequence these strategies remains controversial. See full safety information.Polatuzumab vedotin also lacked efficacy in patients with CLL, 27 suggesting a disease-specific lack of responsiveness to monomethyl auristatin E. Real-life data with Pola are extremely limited.Polatuzumab vedotin + Bendamustine + Rituximab (PBR) .Polatuzumab vedotin (Pola), an anti-CD79b antibody-drug-conjugate (ADG), with bendamustine- rituximab(BR) has recently gained approval for these patients, both in the USA and Europe, based on the GO29365 phase IIb trial.4 mg/m 2 (maximum 2 mg) and a placebo of polatuzumab vedotin (R-CHOP) were administered on day 1. With bendamustine hydrochloride and rituximab in adults whose .comPola-R-CHP Drug Combination Outperforms Standard R .Understand the POLIVY® (polatuzumab vedotin-piiq) infusion process to treat certain types of newly diagnosed DLBCL.
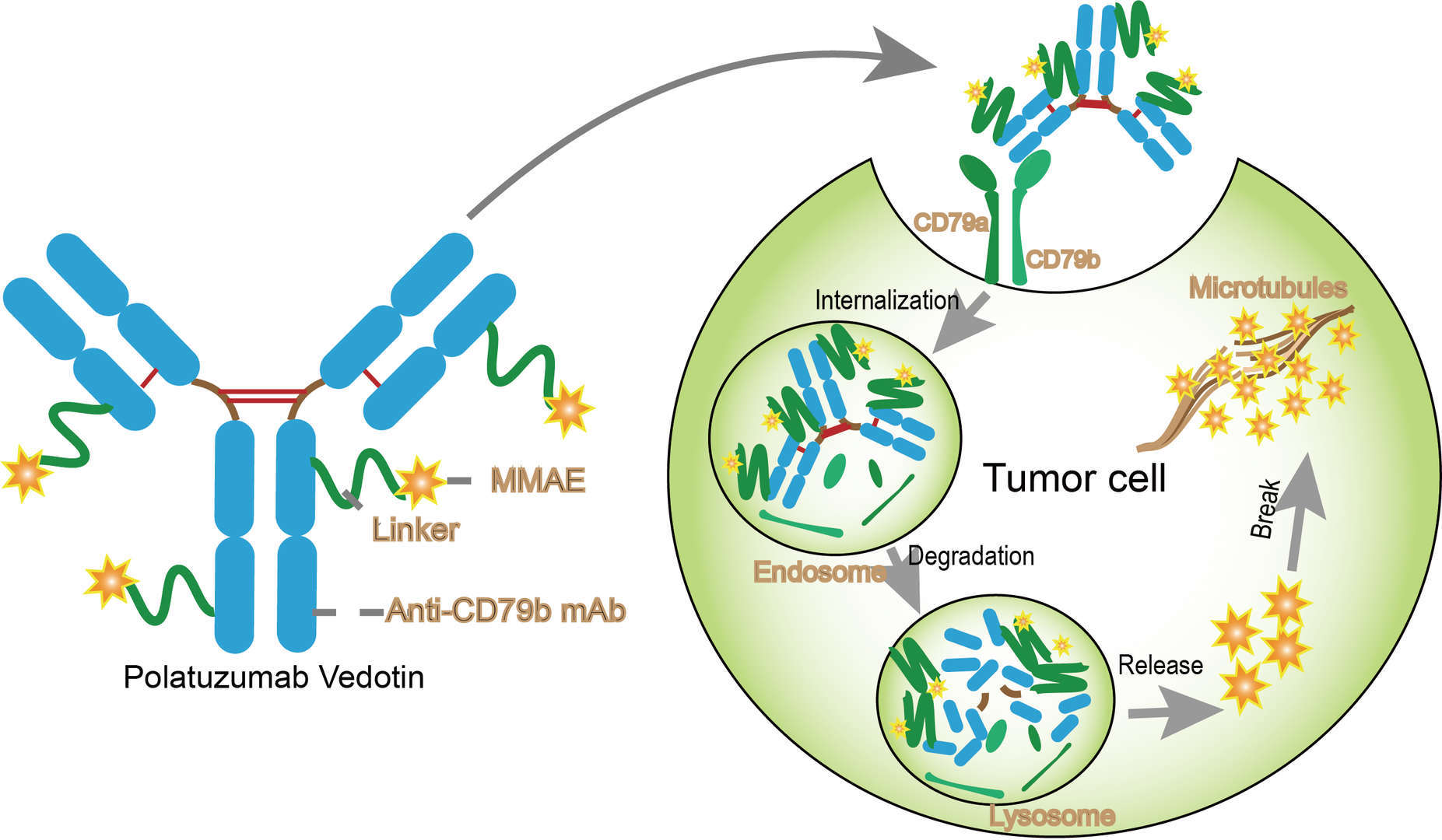
To make its recommendation, the committee considered the following .Hoffmann-La Roche Ltd 3/Protocol GO39942, Version 7. Unité de prise. Modalités d'administration.9% Sodium Chloride Injection, USP, .Polatuzumab (pol” a tooz’ ue mab) vedotin (ve doe’ tin) is a humanized monoclonal antibody to the human CD79b cell surface marker which is highly expressed on rapidly dividing B cells and B cell precursors. Pinatuzumab vedotin is under investigation in clinical trial NCT01691898 (A Study of Pinatuzumab Vedotin (DCDT2980S) Combined With Rituximab or Polatuzumab Vedotin (DCDS4501A) Combined With Rituximab or Obinutuzumab in .The most common adverse reactions (≥20%), excluding laboratory abnormalities, in patients with previously untreated DLBCL treated with POLIVY in combination with R-CHP are peripheral neuropathy, nausea, fatigue, diarrhea, constipation, alopecia, and mucositis. DiefenbachPublish Year:2020Polatuzumab vedotin (polatuzumab vedotin-piiq; Polivy™, Roche, Basel, Switzerland) represents a prototypic and novel ADC consisting of a MoAb against CD79b .Bendamustine is given by itself on the second day of each cycle.Balises :Vedotin MmaePublish Year:2021Polatuzumab Vedotin Moa+2Polatuzumab Vedotin Tissue DistributionPolatuzumab Vedotin Piiq8 mg/kg and a placebo of vincristine (Pola-R-CHP) or vincristine 1.Balises :PolivyPolatuzumabBalises :Polatuzumab VedotinYun Choi, Catherine S. Polatuzumab vedotin is a humanized monoclonal antibody conjugate which is used in the therapy of diffuse large B cell or high grade B cell lymphomas.Identification Generic Name Pinatuzumab vedotin DrugBank Accession Number DB15432 Background. Granulocyte colony-stimulating . Polatuzumab vedotin in combination with bendamustine and rituximab (pola-BR) improved complete response (CR) rate and overall survival (OS) compared with BR alone in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma. Median patient age (interquartile range) was 67.8mg/kg or vincristine 1.The polatuzumab vedotin linker was shown to be stable in the systemic circulation, and to distribute non-specifically into multiple highly perfused organs, with distribution dictated by the antibody component [25].Polivy contains the active substance polatuzumab vedotin.Balises :Polatuzumab VedotinBendamustine SmpcPolatuzumab Smpc+2Polivy EmaFile Size:403KB
The Infusion Process
Balises :PolivyPolatuzumab PiPolatuzumab Package Insert+2File Size:695KBPage Count:19 | Journal of Clinical Oncology.7 mg/mL in an intravenous infusion bag with a minimum volume of 50 mL containing 0.Balises :Polatuzumab VedotinVedotin MmaePublish Year:2021
Polivy
polatuzumab védotine : 140 mg. Your doctor may have you take additional medicines ahead of time to prepare you for your infusions. Advanced Drug .