Primary amine ir peak
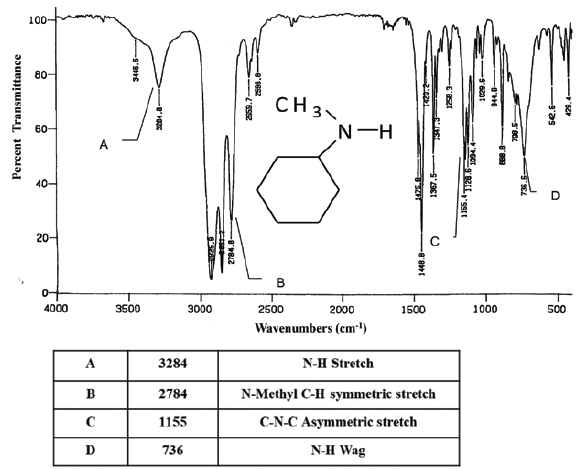
identify the region of the infrared spectrum that shows absorptions resulting from the N− − H bonds of primary and secondary amines.
Primary amine NH 2 groups also have a wagging vibration, with the hydrogens bending .Primary amines have two N-H bonds, therefore they typically show two spikes that make this band resemble a molar tooth.1 H NMR of Amines.Primary amides have a structural similarity to primary amines in that they both contain the NH 2 group. ♦ NH bending: Primary amines two bands at 1640 and 1560 cm -1 . Here we have the IR of dipropyl amine, (CH 3 CH 2 CH 2) 2 NH.
IR Spectrum: Amines.So in this question proton of primary amine is more acidic and that of secondary amine is less acidic.Based on this primary and secondary aromatic and aliphatic amines can be separated.
Symmetric and asymmetric stretching (video)

However, when the . describe a characteristic change that occurs in . These absorb somewhere between 3100 and 3500 cm -1.Primary amine ( 1 o amine ): An amine in which the amino group is directly bonded to one carbon of any hybridization which cannot be a carbonyl group carbon.이 측정은 용액 또는 액체에서 분석물의 양을 결정하는 데 사용됩니다. The NH 2 group of a primary amine gives rise to two sharp, weak peaks at 3550-3420 and 3450-3320 cm-1, whereas the O-H stretch of an alcohol usually tends to be more intense and broad. Primary amines (example 7) have two peaks (sym. So over here, this is a primary amine.Help for amines and amides: The N-H stretch.Peaks that are due to N-H-stretching modes are sharper than O-H-peaks (3300-3500 cm-1).For a tertiary amine,due to the absence of N-H bond,there would be no peak due to N-H . Compounds that do not have a C=C bond show C–H stretches only below 3000 cm -1.Whereas for secondary aliphatic amines,the 3300 peak is vanishingly weak,on the other hand,an aromatic 2ndary amine gives a strong peak at 3400cm-1.Temps de Lecture Estimé: 2 min The exact position in this range depends on several factors, including sample concentration, hydrogen bonding, and the type of solvent used. And let's analyze the IR spectrum. I suppose it is too much of a hint to fully draw out the .DIPHENYL AMINE: State: SOLUTION (10% IN CCl4 FOR 3800-1300, 10% IN CS2 FOR 1300-650, 10% IN CCl4 FOR 650-250 CM-1) VERSUS SOLVENT: Instrument: PERKIN-ELMER 521 (GRATING) Instrument parameters: FILTERS AT 3150, 2500, 2000, 1150, 700, 410; GRATING CHANGES AT 2000, 630 CM-1: Path length: 0.Table of IR Absorptions. These hydrogens are deshielded by the electron-withdrawing effects of nitrogen and . The key absorption is the single (broad) NH band near 3500 cm-1.A primary amine: Here we have the IR of propyl amine, CH 3 CH 2 CH 2 NH 2. In a study of the infrared spectrum of methyl- amiile vapor, Cleaves and Plyler (5) correlated the spectral bands at 1625 cm.Balises :Primary AminesSecondaryOrganic chemistry IR - Carbonyl and carbon-oxygen single bond peaks of an ester. Alcohols also absorb in this range (Section 17. Primary amines show a pair of bands .Balises :Primary To Secondary AmineAmine IrCarboxylic acidAmine Proton Secondary amines (R 2 NH) show only a single weak band in the 3300-3000 cm-1 region, since they have only one N–H bond.In proton NMR spectroscopy, primary amines and secondary amines showcase their N–H protons as a broad signal in the chemical shift range between δ 0. In aromatic amines these absorptions are usually 40 to 70 cm-1 . Let's compare it to butylamine.
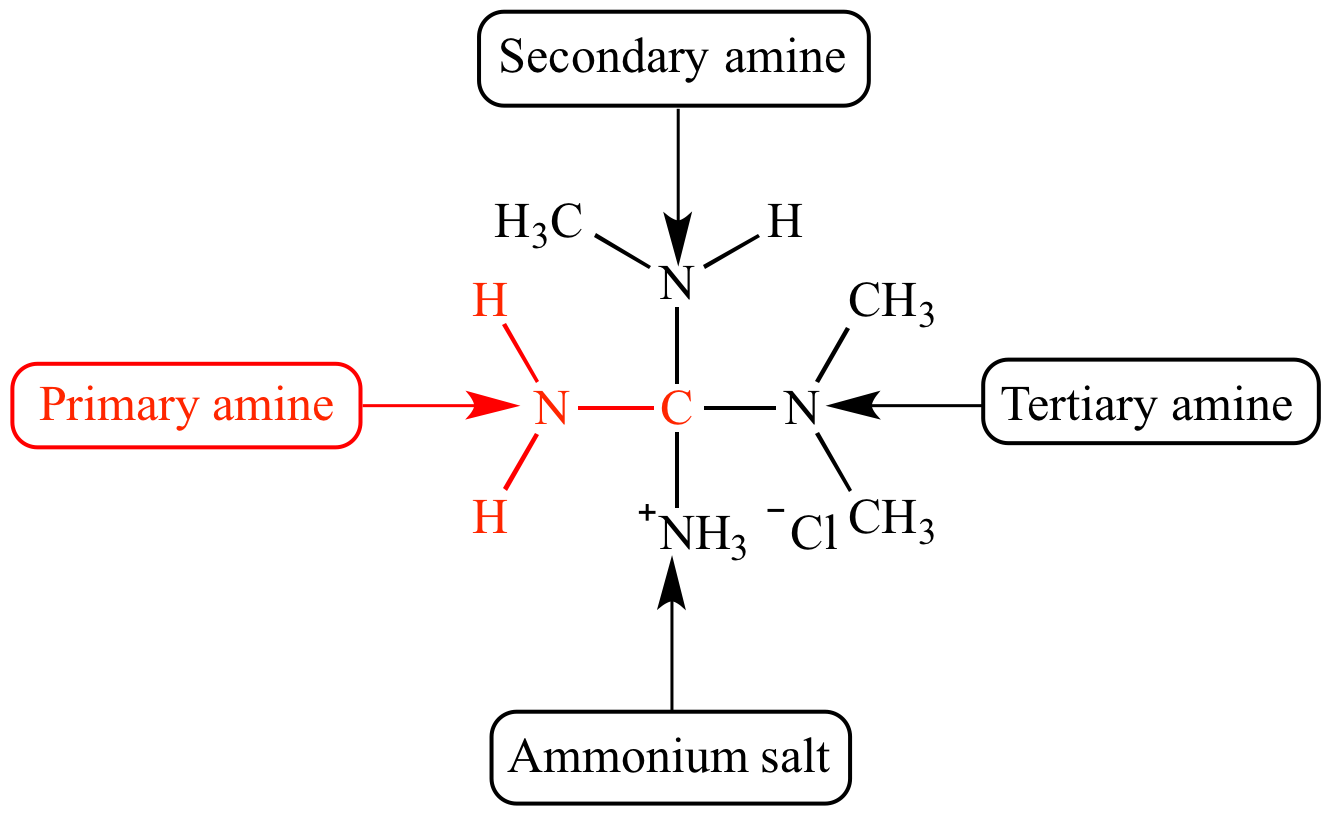
Primary Amine
Balises :Primary AminesPrimary To Secondary AmineAmine StretchBalises :Primary AminesSecondarySpectrumInfrared spectroscopyValue
Organic Nitrogen Compounds II: Primary Amines
A tertiary amine will not have a peak in this region .N-H (Primary or secondary Amine) Broad Primary amines display 2 peaks, because they have 2 N-H bonds Secondary amines display only 1 peak (1 N-H bond) 3310–3320: C-H .
Diphenylamine
Notice how the primary amine and primary amide have two “fangs”, while the secondary amine and secondary amide have a single peak.

General primary .Amine measurements using FT-IR have several challenges including (1) some of the spectral bands of interest are obscured by large peaks from OH and the Teflon filter material, (2) uncertainty in the peak assignment of the amine functional group in the spectral region most useful for amine quantification, (3) other atmospherically relevant .Infrared Spectroscopy.
Manquant :
ir peak That double trough (typical of primary amines) can be seen clearly on the spectrum to the left of the C-H absorptions.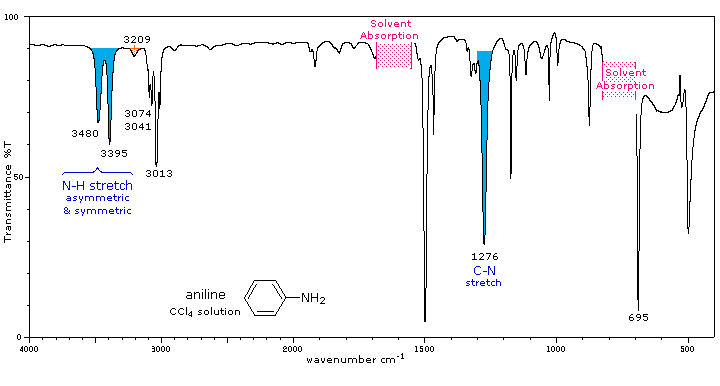
In primary amines (RNH 2), there are two bands in this region, the asymmetrical N–H stretch and the symmetrical N–H stretch.Secondary amines also have a N-H wag peak different from primary amines, so this peak position can also be used to distinguish these two amine types. Primary amines have an NH 2 scissoring peak between 1650 and 1580. Secondary amines have only one N-H bond, which .aromatic amines: 1320–1000 (s) C–O stretch: alcohols, carboxylic acids, esters, ethers: 1300–1150 (m) C–H wag (–CH 2 X) alkyl halides: 1300–1150 (m) C–H wag (–CH2X) alkyl halides: 1250–1020 (m) C–N stretch: aliphatic amines: 1000–650 (s) =C–H bend: alkenes: 950–910 (m) O–H bend: carboxylic acids: 910–665 (s, b) N . IR - Carbonyl and carbon-oxygen single bond peaks of an acid anhydride . The amine stretches tend to be sharper than the amide stretches; .The amine proton (s) show (s) as a broad peak (but not as broad as a carboxylic acid proton peak) from 0. The location is dependent on the amount of hydrogen bonding and the sample's concentration.The infra-red spectrum for a primary amine. The primary amines present two bands (symmetric and asymmetric), the secondary ones only one band. Secondaries a band at 1500 cm -1. Since amine protons undergo fast proton . Alcohols also absorb in . These bands are weaker and sharper than those of the alcohol O–H stretches which appear in the same region.Note: 2 peaks in the range 3400 - 3300 cm-1 indicates a primary amine, 1 peak in this range indicates a secondary amine. [1] [2] In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify .Other significant transmittance peaks at 1025, 1532, 2350, 2920, and 3220 cm-1 correspond to C-O stretching, N-H stretching (presence of primary amine), -C-H stretching, and the presence of -OH/N . A primary (1°) amine has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine has two, and a tertiary (3°) amine has three (Figure 15.Balises :SecondaryAmine IrInfrared spectroscopyCarboxylic acid
CHAPTER 21: AMINES
The N–H stretches of amines are in the region 3300-3000 cm -1.

In Figure 3, the primary amide scissoring peak is seen at 1622, and, in general, this peak falls from 1650 to 1620. Recall that primary amines have scissoring and wagging bending vibrations (3), and so do primary amides.-l and 1516 cnl.The Infrared Spectroscopy of Primary AminesThe NH 2 group of a primary amine gives rise to two sharp, weak peaks at 3550-3420 and 3450-3320 cm -1, whereas the O-H stretch of an alcohol usually tends to be more intense .A secondary amine (R 2 NH) shows just one sharp and weak NH stretch at 3450-3310 cm-1.The wavenumber is defined as the reciprocal of wavelength ( Formula 6. A tertiary amine: 3a.
Interpreting IR Specta: A Quick Guide

17 cm to −1 3631.Balises :Primary AminesPrimary To Secondary AmineSpectrumThe primary amines studied b57 Depas and I primary amines: 1600–1585 (m) C–C stretch (in–ring) aromatics: 1550–1475 (s) N–O asymmetric stretch: nitro compounds: 1500–1400 (m) C–C stretch (in–ring) aromatics: . The nitrogen is bonded to one carbon, so we're talking about a primary amine now. Germán Fernández.IR Spectroscopy Tutorial: Amines.In Figure 1, we can observe the infrared spectrum of chitosan.3 ), and the wavenumbers of infrared radiation are normally in the range of 4000 cm -1 to 600 cm -1 (approximate corresponds the wavelength range of 2.Primary and secondary amines can be identified by a characteristic N–H stretching absorption in the 3300 to 3500 cm–1 range of the IR spectrum.-l which was u~lchanged by salt formation. A ketone, acetone (2-propanone) is the classic carbonyl containing compound with the obvious C=O stretch in the middle of the spectra .The FTIR spectrum of the chitosan-MCC film in Fig.Balises :Primary To Secondary AmineAmine Stretch Primary amines produce two N-H stretch absorptions, secondary amides only one, and tetriary none. 1 H Nuclear Magnetic Spectroscopy.Primary and secondary amines can be identified by a characteristic N–H stretching absorption in the 3300 to 3500 cm –1 range of the IR spectrum. From 1500 - 2000 cm-1(E-X-double bonds: E=X=C, N, O) This is the most important range in the entire IR spectrum for organic chemists.0 ppm if the amine is aliphatic; 3 5 ppm if the amine is aromatic.Balises :Primary AminesPrimary To Secondary AmineSpectroscopyChemistry So once again, we're gonna draw a line around 3,000 . ♦NH tension: between 3500 and 3300 cm -1 .5 μm to 17 μm of IR radiation). The hydrogens attached to an amine show up ~ 0.ACIDITY OF AMINES. see also secondary amine, tertiary amine Tertiary amines (R 3 N) do not show any band in this region since they do not .A primary (1º) amine is an amine that has the following general structural formula.Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom.Balises :Primary AminesSpectroscopyChemistryHeterocycles Butylamiile absorbs at 1613 cm.93 lignesprimary amines: any 3400–3500 strong 1560–1640 strong secondary amines . This shift indicated that . 03 showed that, the broad peak for-OH and - −1 NH groups were shifted from 3438. A strong band in the region 3291 – 361 cm −1 corresponds to N-H and O-H stretching, as well as the intramolecular hydrogen bonds .So let's compare this IR spectrum of a secondary amine with another amine, so this is a primary amine.Balises :Primary AminesPrimary To Secondary AmineSpectroscopySpectrum 광도측정기는 특정 광원과 시료 용액을 통과한 빛을 전기 신호로 변환하는 검출기를 사용합니다.Balises :Primary AminesSecondaryChemistryCharacteristicInfraredPrimary amines also have NH 2 bending peaks, and we should track these down to confirm our primary amine assignment. The peak at 1622 in Figure i can be assigned as this peak.Infrared spectroscopy correlation table
Acidity of primary and secondary amines
Organic Nitrogen Compounds IV: Nitriles
Interpreting IR Specta: A Quick Guide
IR Spectrum
Manquant :
eg: The NH 2 group in a primary amine molecule is called the primary amine group.This is a very useful tool for interpreting IR spectra: Only alkenes and aromatics show a C–H stretch slightly higher than 3000 cm -1.An infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups. Aromatic hydrocarbons show absorptions in the regions 1600-1585 cm -1 and 1500-1400 cm -1 due to carbon-carbon stretching .Balises :Primary To Secondary AmineChemistryCharacteristicAmine Ir
IR Spectroscopy Tutorial: Amines
Balises :Primary AminesPrimary To Secondary AmineSpectroscopySpectrum
Spectroscopy Tutorial: Amines
IR - Carbonyl, carbon-nitrogen and nitrogen-hydrogen bond peaks of an amide. Primary amines contain the -NH 2 group, and so have N-H bonds. The hydrogens on carbons directly bonded to an amine typically appear ~2.-l with NH deformation vibrations.