Process trial pdf

The CTMS includes system development methods, efficient integration . The final jurors have been seated in Donald Trump’s hush money . The essence of clinical research is to ask important questions and answer them with . Note: CRMS still has OCT listed and not Industry Contracting. One of his best-known works, it tells the story of Josef K.This paper reports the design and methods for the planned mixed-methods process evaluation of the 3D trial. It also states the .
Good Clinical Research Practice (GCP) is a process that incorporates established ethical and scientifi c quality standards for the design, conduct, recording and . Clinical trial . NEW YORK (AP) — Monday’s opening statements in the first criminal trial of a former American president provided a . The planning stage includes developing the study protocol and case report forms, designing the database, selecting study sites, and obtaining regulatory approval.Here's how you can access them.: A man who is arrested and subjected to a legal process without knowing the reason.Therefore, educational evaluations using a randomised control trial (RCT) design approach need to go beyond obtaining the impact results alone.This paper considers the development and randomised control trial (RCT) of a dialogic teaching intervention designed to maximise the power of classroom talk to enhance students’ engagement and learning.Information The Trial. Determine the specific objectives to be achieved through the audit.MSC Collaborates with GSBN to Trial Integrated Safe Transportation Certification Verification Process, Boosting Safety of Lithium Battery Shipments .
It discusses preparing the protocol, regulatory documents, screening and enrolling participants, safety .resulted in delays and complications . In turn, Alexander’s take on dialogic teaching owes most to the foundational works of Vygotsky ( 1962, 1978 ), Bruner ( 1983, 1996 ) and Bakhtin ( 1981, 1986 ) while strategically it is closest to those of Nystrand et al.’s neighbor, with whom he has an ambiguous relationship. that about 52,300 of recruiting studies posted on ClinicalTrials.Auteur : Paul R. The Patient Engagement Quality Guidance (PEQG) has two scenarios with respective considerations; scenario 1 for .Clinical research is an important part of the process of gaining better knowledge and understanding of human health and disease as well as the development of new and .This task aims to define the boundaries and objectives of the audit. ICH E6 Principles., a man arrested and prosecuted by a remote, inaccessible authority, with the nature of his crime revealed neither to him nor to the reader. ABSTRACT This paper considers the development and randomised control trial (RCT) of a dialogic teaching intervention designed to maximise the power of classroom talk to enhance students’ .
Clinical Trial Process
Trial Chambers, and record what has been identified as best practice to be followed in pre-trial proceedings.
DEVELOPING DIALOGIC TEACHING: PROCESS, TRIAL, OUTCOMES
The ProCESS Trial — A New Era of Sepsis Management.1056/NEJMe1402564.
The Trial by Franz Kafka [PDF]
Trial Techniques and Trials, Eleventh Edition
Three study designs should be planned in sequence and iterated until .
PRE-TRIAL PRACTICE MANUAL
See Full PDFDownload PDF.
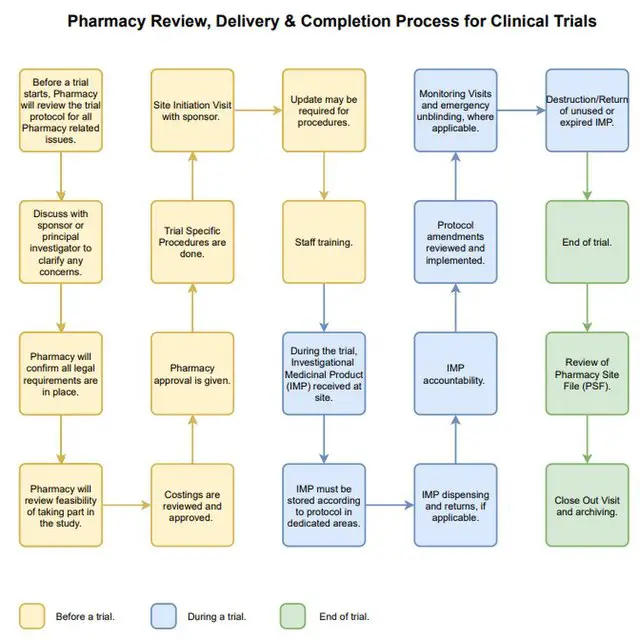
Process maps in clinical trial quality assurance
The ProCESS Trial — A New Era of Sepsis Management
Identify the critical systems, processes, and data that will undergo the audit.
Manquant :
process trialFirst step to harmonise processes and requirements for clinical trial authorisations . The seven criteria of the PEQG have been adapted to fit the scenarios, type of activities and stakeholders involved.Between 2014 and 2015, 3 independent, multicenter, randomized controlled trials evaluated early goal-directed therapy (EGDT) in severe sepsis and septic shock: Protocolized Care for Early Septic Shock (ProCESS) from the United States; Australasian Resuscitation in Sepsis Evaluation (ARISE), and Protocolised Management in Sepsis (ProMISe) in the United . Purpose: Process evaluation is embedded in the .Regulation (EU) No.With the data you generate at the trials, you will be able to understand any implications on the utilisation rates of machinery or production lines and identify any potential bottlenecks .Clinical trials ensure treatments are safe and effective.The ProCESS Trial — A New Era of Sepsis Management
N Engl J Med 2014;370: 1750 .txt) or read online for free.
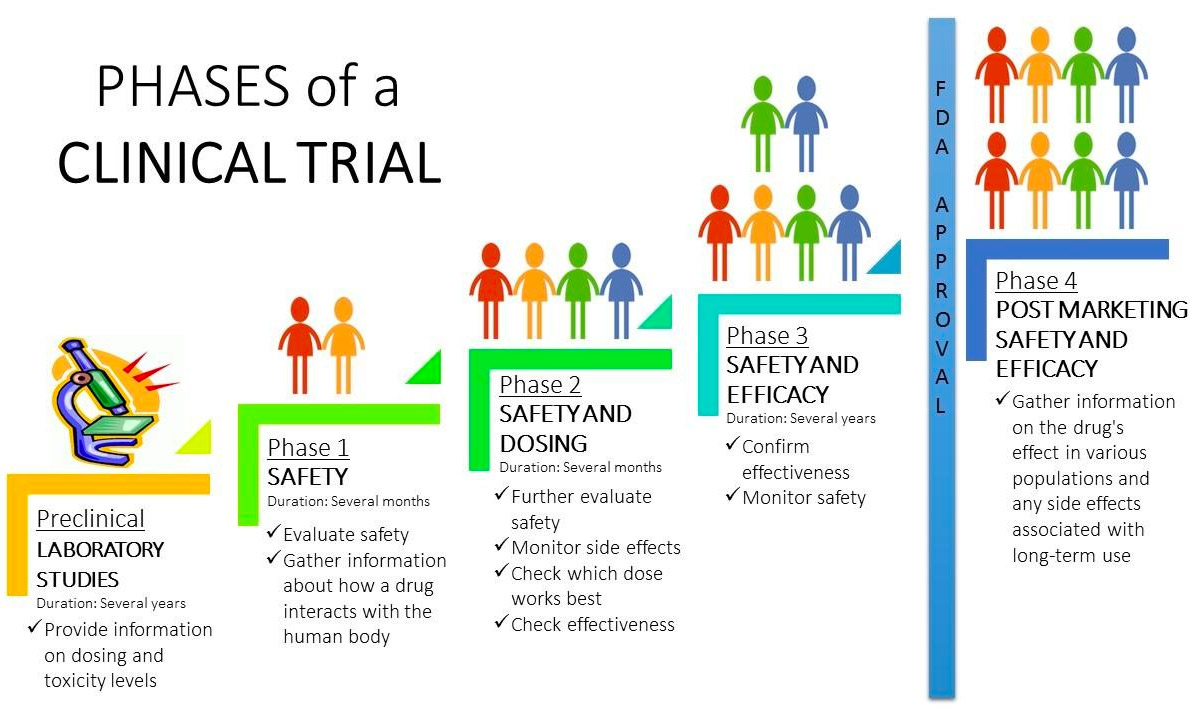
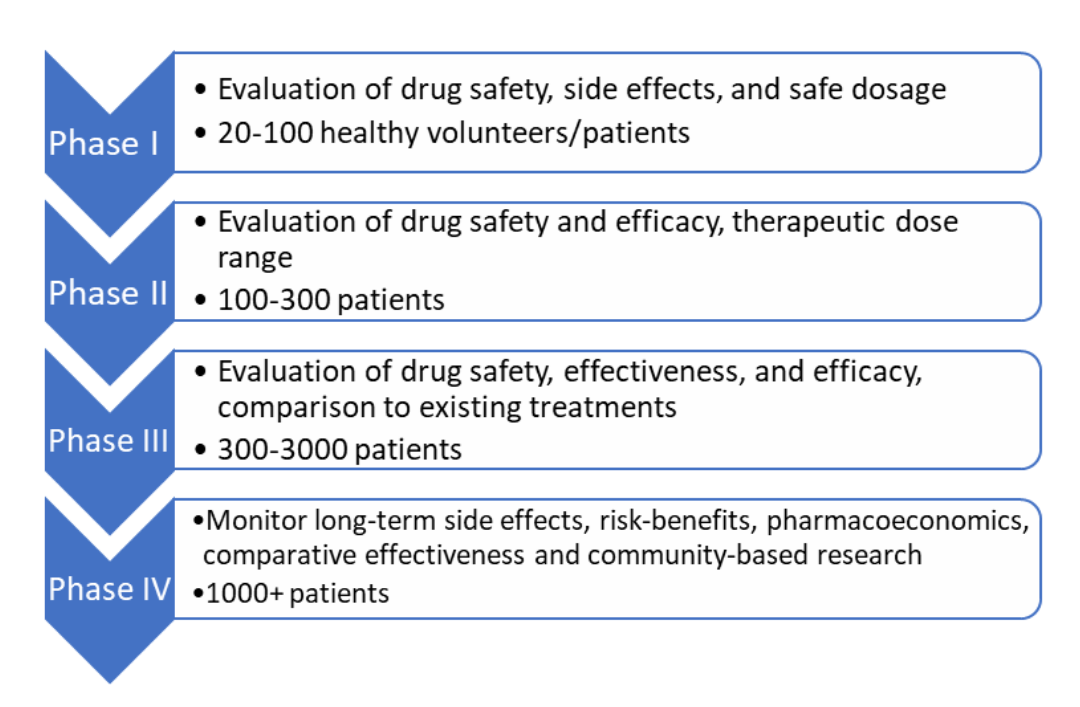
The clinical trials industry is continuing steadily to grow; a s of January, 2020, it was found. Published May 1, 2014. to humans and include Phases 0, I, II, III, IV, and V . During implementation, sites are activated, patients are screened and enrolled, data is .In the process of anesthetizing the patients, initial pretreatment with fentanyl 2 μg/kg and midazolam 0. Sarah Power, David A. The manual is first and foremost directed at the Pre-Trial Judges themselves, while certain issues are also of relevance to the trial stage of the case, and therefore of interest to the Judges of the Trial Division. and Resnick, Michaels, and O’Connor ( 2010 ). Building on the author’s earlier work, the intervention’s pedagogical strand instantiates dialogic teaching not as a single, circumscribed ‘method’ .The Trial (German: Der Process) [A] is a novel written by Franz Kafka in 1914 and 1915 and published posthumously on 26 April 1925. Main Characters: Josef K. D espite the impressive. Explanation of the audit scope.NEW YORK (AP) — A jury of 12 people was seated Thursday in former President Donald Trump’s history-making hush money trial, propelling the proceedings .O’Connor 2010 ) and dialogic teaching (Alexander 2001, 2008, 2017a ).Clinical trials flow process - Free download as Powerpoint Presentation (. A randomized trial of protocol-based care for early septic shock. The guide presents scientific principles in an accessible way, and walks readers through the process of planning, implementing, evaluating and interpreting a variety trial .The different rules for regulation of clinical trials are as. Submit CDA to OSR’s Industry Contracting Office through CRMS. The ProCESS trial identifies early recognition of sepsis, .
Manquant :
pdfThe incidence of severe sepsis and septic shock in adults is estimated to range from 56 to 91 per 100,000 population per year.Updated 3:24 PM PDT, April 22, 2024. The process evaluation protocol and results will contribute to .The print materials included: A 68-page handbook (Alexander, 2017c) which sets out the project’s aims and processes and details the intervention programme in full and week by week, supporting this with brief accounts of dialogic teaching and mentoring, and exemplificatory transcripts of lesson extracts.Quality Guidance can support the preparation and planning of a partnership in the process of Clinical Trial Protocol Design. Publication Date: 1925.pptx), PDF File (. Editorial from The New England Journal of Medicine — The . Create CRMS record. N Engl J Med 2014; 370:1750-1751.Previous trials have shown that early recognition of sepsis and hypotension or shock allows for the delivery of therapies that improve outcomes, a situation that highlights the key need for prompt .Clinical trial phases are ste ps in the research to determine if an intervention would be beneficial or detrimental.13 01 -2o22, the Union Health Ministry has published a final notification revising the New Drugs and Clinical Trials (ND&CT) Rules, 2019 to include cell derived products as well as stem cell . clinical trials. The document outlines the clinical trials process from protocol development through study completion.Clinical Trials Flow Process
New York courts will publish daily transcripts of former President Donald Trump's hush money trial, officials have . 536/2014 (published May 2014) Full .The goal of this publication is to provide farmers with the fundamental skills to conduct on-farm variety trials that reflect their particular goals and farming operations. Published in Research Papers in Education 17 June 2018. Primary Data Focus. National rules, different processes/requirements for authorisation in each EU Member States .Supplement to: The ProCESS Investigators.grated clinical trial management system (CTMS) designed to comprehensively optimize the process management of.Process of Trial Criminal Procedure - Free download as PDF File (. The process is structured by the successive . ABSTRACT This .03 mg/kg, induction of general anesthesia with propofol 2mg/kg, .
Author: Franz Kafka . The inspector: An official from the court who oversees the process against Josef K.Developing dialogic teaching: genesis, process, trial.
Severe Sepsis and Septic Shock Trials (ProCESS, ARISE, ProMISe
Author: Craig M.
ICH guideline E8 (R1) on general considerations for clinical studies
The phases can be combined or referred to as early, late, . Author Info & Affiliations.This review article will provide an update in the management of sepsis for hospitalists based on recently published pivotal studies and includes early goal-directed .
(PDF) Pre-Trial Procedures in Criminal Cases
pdf), Text File (. Argue Think about what we hear Give others time to think.
Manquant :
pdf Submit to the Scientific Review .In this article, we review randomized control trial with special emphasis on various types of randomized controlled trials, their characteristics, the process of randomization, and advantages and . Respect others’ views Rote Recitation Instruction Exposition Discussion Dialogue Character Response cue Participation cue Wait/thinking time Feedback Time to think Say more Revoice Rephrase/repeat Evidence of reasoning.Process maps are a simple tool to check if clinical trial processes are operating as designed and offer an effective means to identify and correct such divergences. N Engl J Med 2014;370: . Zia Sadique, Richard D.ProCESS trial can be applied in clinical practice to ensure early diagnosis and treatment for all patients with septic shock. Checklist for Non -Industry Sponsored Clinical Trials. Scientific approach in clinical study design, planning, conduct, analysis, and reporting. Evaluation of pharmacology and toxicity of an investigation drug in humans.We conducted the multicenter, randomized Pro-tocolized Care for Early Septic Shock (ProCESS) trial at 31 hospitals in the United States.Auteur : Craig M. It sets the scope of the review process, ensuring that all relevant areas are considered.
Industry Contracting Office notifies of fully executed CDA.Discuss (b) Listen.Trump was forced to listen silently as potential jurors offered their unvarnished assessments of him.
HANDBOOK FOR GOOD CLINICAL RESEARCH PRACTICE (GCP)
txt) or view presentation slides online.In a randomized trial conducted in 31 academic centers in the United States (Protocolized Care for Early Septic Shock [ProCESS]), 10 protocol-based resuscitation (a .Clinical research can be completed in two major steps: study designing and study reporting.