Propane dehydrogenation review
Pt-based catalysts have been widely proven as a kind of efficient propane dehydrogenation catalysts due to their affinity for paraffinic C–H bonds and been applied into UOP Oleflex technique [30, 31].Nonoxidative propane dehydrogenation (PDH) produced 13. Pt- and CrOx-based catalysts are widely applied in commercialized PDH processes, and both exhibit high activity and propylene yields. Jump to site search . 13 In general, due to a very large number of available data and sometimes contradictive statements in different studies, no statistically proven relationships between .Dehydrogenation of Propane and n-Butane Catalyzed by Isolated PtZn4 Sites Supported on Self-Pillared Zeolite Pentasil Nanosheets.Propane from shale gas is converted commercially to propylene through propane dehydrogenation (PDH) (2–4), but the reaction is highly endothermic . Under suitable conditions, the supported Pt–Sn and CrOx catalysts widely used in Recent Advances in . Common semiconductors with low cost and . Co1Ni1P displayed higher propylene selectivity than Co2P . An Author Correction to this article was published on 06 January 2022. On both the flat Pt(111) and the stepped Pt(211), the coincidence of the two corresponding potential energy diagrams indicates that propane dehydrogenation shows no particular preference for any of the two reaction pathways. used high a h-BN and low h-BN content catalyst.The oxidative dehydrogenation of propane is an attractive reaction for propylene production, but the overoxidation leads to low propylene selectivity at considerable propane conversions.In the current review, direct and CO 2-assisted dehydrogenation of propane has been compared from three aspects: reaction mechanisms, catalyst compositions, .A brief analysis of the oxidative propane dehydrogenation to propene over V-based catalysts is given in a review published in 2018 and dealing with aerobic oxidations of C 1 –C 4 alkanes.1 Propane dehydrogenation. In this work, we investigate bimetallic CoXNi2-XP as an alternative, non-noble metal propane dehydrogenation catalyst.In the current review, direct and CO 2 -assisted dehydrogenation of propane has been compared from three aspects: reaction mechanisms, catalyst compositions, and CO 2 footprint analysis.The focus of this critical review is on the dehydrogenation of propane.Commercial PDH processes utilize either . On the basis of adsorption energies, propane is physisorbed on Ni(111) surface, whereas propylene exhibits chemisorption .
Dehydrogenation of light alkanes to mono-olefins
Propane dehydrogenation has been a promising propylene production process that can compensate for the increasing global demand for propylene.Furthermore, the review also emphasizes advanced synthetic strategies based on novel design of PDH catalysts with improved dehydrogenation activity and stability.current review.
In the current review, direct and CO 2 -assisted .The increasing demand for short chain olefins like propene for plastics production and the availability of shale gas make the development of highly performing . Research into propane dehydrogenation is an ever-growing field and to date there are several review articles available on direct dehydrogenation (DDH) .In this critical review, we examine the significant advances made in the development of dehydrogenation catalysts, evaluating the technological and environmental merits.
Advances in zeolite-supported metal catalysts for propane dehydrogenation
This review focuses on the CO 2-ODH of propane over zeolite and metal .
As the average CO 2 emissions from electricity generation decrease due to the utilization of renewable energy, CO 2 -ODHP potentially .
Propane Dehydrogenation
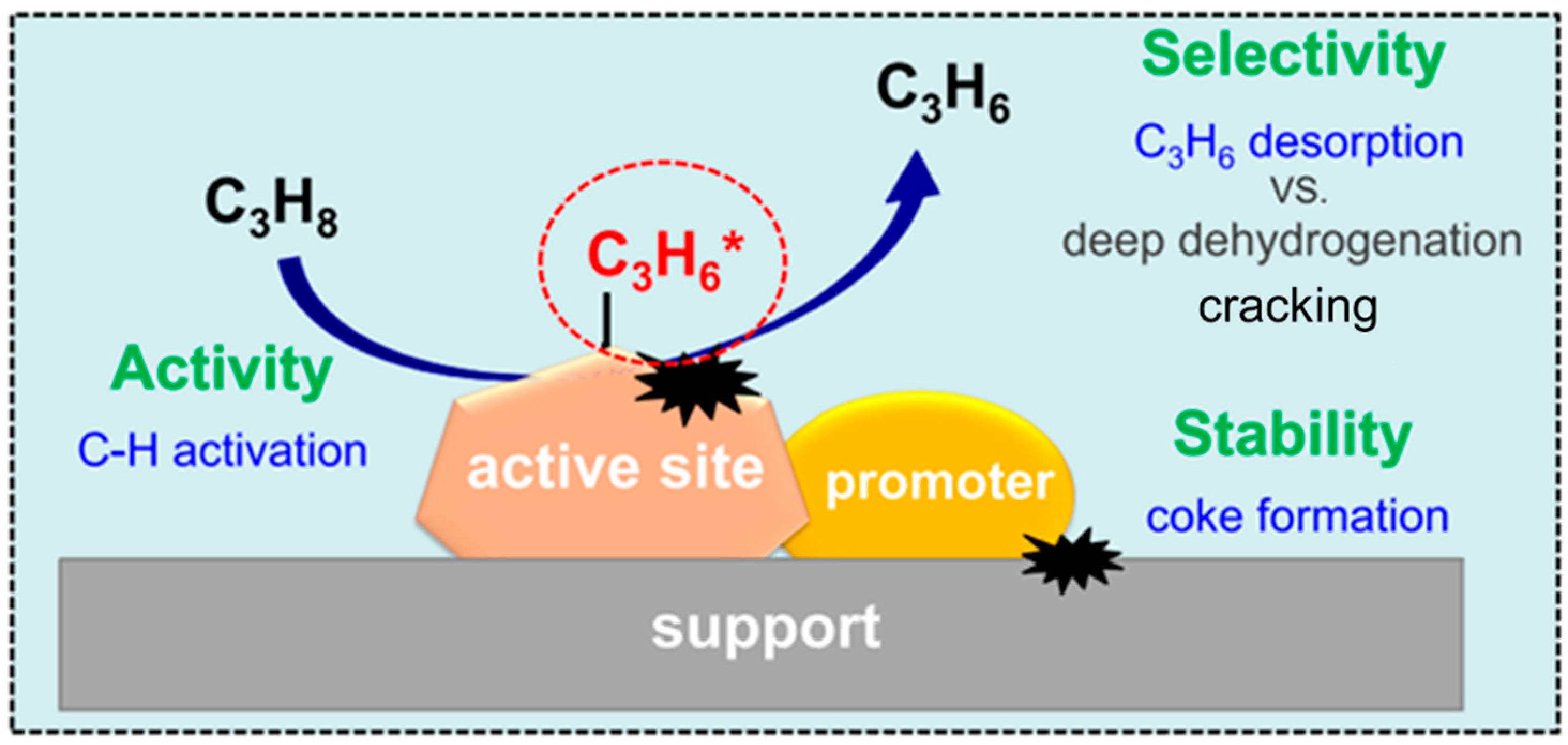
Propane dehydrogenation (PDH) is an industrial technology for direct propylene production, which has received extensive attention and realized large-scale application.

Direct and oxidative dehydrogenation of propane: from . Dehydrogenation of propane (PDH) technology is one of the most promising on-purpose technologies to solve supply-demand unbalance of propylene.Propane dehydrogenation (PDH) is one of the most promising on-purpose technologies to produce propylene, and thus has attracted widespread attention from both the acad Synthesis, modification and tailoring of properties of nanoporous materials 2022 Inorganic Chemistry Frontiers Review-type Articles Jump to main content .Review Ab Initio Multiscale Process Modeling of Ethane, Propane and Butane Dehydrogenation Reactions: A Review Luka Skubic 1, Julija Sovdat 1, Nika Teran 1, Matej Huš 1,2, Drejc Kopacˇ 1,* and Blaž Likozar 1,* 1 National Institute of Chemistry, 1000 Ljubljana, Slovenia; lukaskubic@hotmail. Reaction of the hydrogen with oxygen in the sweeping gas to form water generated heat to help drive the endothermic dehydrogenation reaction.70 kcal/mol), requiring temperatures that may .5% conversion of propane and 94.This review describes recent advances in the fundamental understandings of the PDH process in terms of emerging technologies, catalyst development and new . Propane dehydrogenation (PDH) has attracted widespread attention in recent years .The direct dehydrogenation of propane (DDP) is thermodynamically limited and is highly endothermic (Δ H° r = 29. ACS Catalysis 2022 , 12 . The commercial PDH technologies .Propane dehydrogenation to propene is one reaction for which the intermediate temperature range is particularly suited, and Pt,Sn/Mg .Among all the methods, oxidative dehydrogenation of propane (ODHP) to propylene appears highly prospective due to its thermodynamic leverage, and could proceed at lower temperature in the presence of an oxidant (limited oxygen) [2]. Here, we report the oxidative dehydrogenation of propane by oxygen in the presence of hydrogen chloride.Direct dehydrogenation of propane (DDHP) offers high propylene selectivity but is limited by quick deactivation due to coke formation.
Propane and Butane Dehydrogenation Reactions: A Review
comPropane dehydrogenation: catalyst development, new . Compared with the traditional catalytic .The superiority of boron-based (B-based) catalysts in inhibiting olefin peroxidation has shed light on the industrialization landscape of the oxidative dehydrogenation of propane (ODHP) technology.Propylene, a primary building block in the chemical industry, is mostly produced through the stream cracking of naphtha (), an energy-intensive process that relies on petroleum feedstocks.The initial propane conversion decreases as the steaming temperature rises, indicating that the dealumination of the HZSM-5 support has a significant effect on the . The major roles of soft oxidants include inhibiting overoxidation and improving propylene .Catalyst design and tuning for oxidative dehydrogenation .Propane dehydrogenation to produce propylene follows a two-step reaction mechanism, with the formation of 1- or 2-propyl.Highly Effective Direct Dehydrogenation of Propane to . However, Pt-based catalysts with high stability at ≥600 °C have barely been reported because the catalysts typically result in short catalyst life owing to side reactions and coke formation. Thermodynamic equilibrium analysis of propane dehydrogenation with carbon dioxide and side . Currently, non-oxidative direct dehydrogenation (DDH) is the only commercialised process, and this is reflected in the high space–time yield commonly .On-purpose technologies such as propane dehydrogenation (PDH) thus became very profitable, due to the gap between propylene production and demand and the difference . Propane dehydrogenation (PDH), an “on purpose” propylene production technology is developing.Our contribution demonstrates that rhodium, an element that has barely been reported as an active metal for selective dehydrogenation of alkanes becomes a very active, selective, and robust dehydrogenation catalyst when exposed to propane in the form of single atoms at the interface of a solid-supported, highly dynamic liquid Ga–Rh mixture. The discovery of earth-abundant and environmentally friendly metal with high activity in C–H cleavage is of great importance.The thermodynamics of ODHP under different modes of operation has been discussed in a . The oxidative dehydrogenation of propane, primarily sourced from shale gas, holds promise in meeting the surging global demand for propylene. However, the selectivity of the C3H6 product is always poor because of overoxidation.Oxidative dehydrogenation of propane (ODHP) as an exothermic process is a promising method to produce propene (C3H6) with lower energy consumption in chemical industry.Oxidative propane dehydrogenation (OPDH) is an emerging technology in which thermodynamically driven overoxidation of propane to carbon oxides (CO x) . This article has been updated. They studied the reaction mechanism of oxidative propane dehydrogenation (OPDH) using kinetic modeling and DFT calculations, proposing a detailed reaction pathway yielding various products in OPDH.6 million metric tons of propylene in 2019, accounting for ~11% of global propylene production (). The present review highlights the promising PEC-WS process for overcoming energy and environmental issues and essential strategies in developing photoanodes.govRecommandé pour vous en fonction de ce qui est populaire • Avis
Efficient conversion of propane in a microchannel reactor at
Review Comparison of direct and CO2-oxidative dehydrogenation of propane
orgRecommandé pour vous en fonction de ce qui est populaire • Avischemistry-europe. prepared vanadium-based CaO-γ-Al 2 O 3 supports for the oxidative dehydrogenation of propane [ 71 ].Propene is an important chemical raw material with continuously growing demand from the propene downstream industry. The oxidative dehydrogenation of propane using CO 2 (CO 2 -ODP) is a promising technique for high-yield propylene . Co species encapsulated within zeolite are highly efficient for catalyzing direct . & Sahebdelfar, S.Unraveling the structure-activity relationship and improving the catalytic performance is paramount in propane dehydro-aromatization reactions.Here, propane dehydrogenation (PDH) to propylene and side reactions, namely, cracking and deep dehydrogenation on Ni(111) surface, have been theoretically investigated by density functional theory calculation. However, owing to the complexity of the structure and reaction network of B-based catalysts, systematic understanding of the .However, Pt-based catalysts suffer from deactivation caused by coke formation, resulting from C C .

CeO2 was found to be an .Qiang Chen, in Chemical Engineering Journal, 2022.Propane dehydrogenation (PDH), an “on purpose” propylene production technology is developing.3390/catal10121405 Corpus ID: 229436743; Ab Initio Multiscale Process Modeling of Ethane, Propane and Butane Dehydrogenation Reactions: A Review @article{Skubic2020AbIM, title={Ab Initio Multiscale Process Modeling of Ethane, Propane and Butane Dehydrogenation Reactions: A Review}, author={Luka Skubic and Julija . Pt- and CrO x -based catalysts are widely applied in commercialized PDH . Herein, the ODHP reaction into C3H6 on a model rutile(R)-TiO2(110) surface at low .
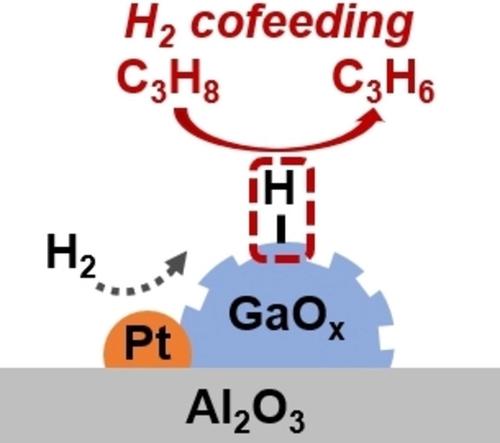
In this context, NiGa nanoalloys generate hydrogen shields that .ConspectusPropylene serves as one of the most significant compounds in the chemical industry.










