Properties of graphite mineral

Mineral
Graphite is a form of pure carbon that normally occurs as black crystal flakes and masses.Diamonds are one of the most precious and valuable gemstones in the world, known for their exceptional hardness, brilliance, and durability. The word graphite is derived from the Greek word graphein, which means, to write. Energy & Fuels 2022 , 36 (15) , 8256-8266. The nonmetallic properties include inertness, high thermal resistance, and . Minéral natif. It is also chemically inert and highly refractory. It has important properties, such as chemical inertness, thermal stability, high electrical conductivity, and lubricity (slipperiness) that make it suitable for many industrial .Graphene is, basically, a single atomic layer of graphite, an abundant mineral that is an allotrope of carbon that is made up of very tightly bonded carbon atoms organized into a hexagonal lattice. Physical Properties of Graphite. Mineral fracture surfaces may be rough, uneven, or show a conchoidal fracture. Graphite is a good conductor of electricity due to its free delocalized electron which is free to move . 1 illustrates the schematic representation of the thermal properties measurement for asphalt binders. Graphite has a giant covalent structure in which: each carbon atom forms three. Turmoil in battery metal markets led the cost of Li-ion battery packs to increase for the first time in 2022, .
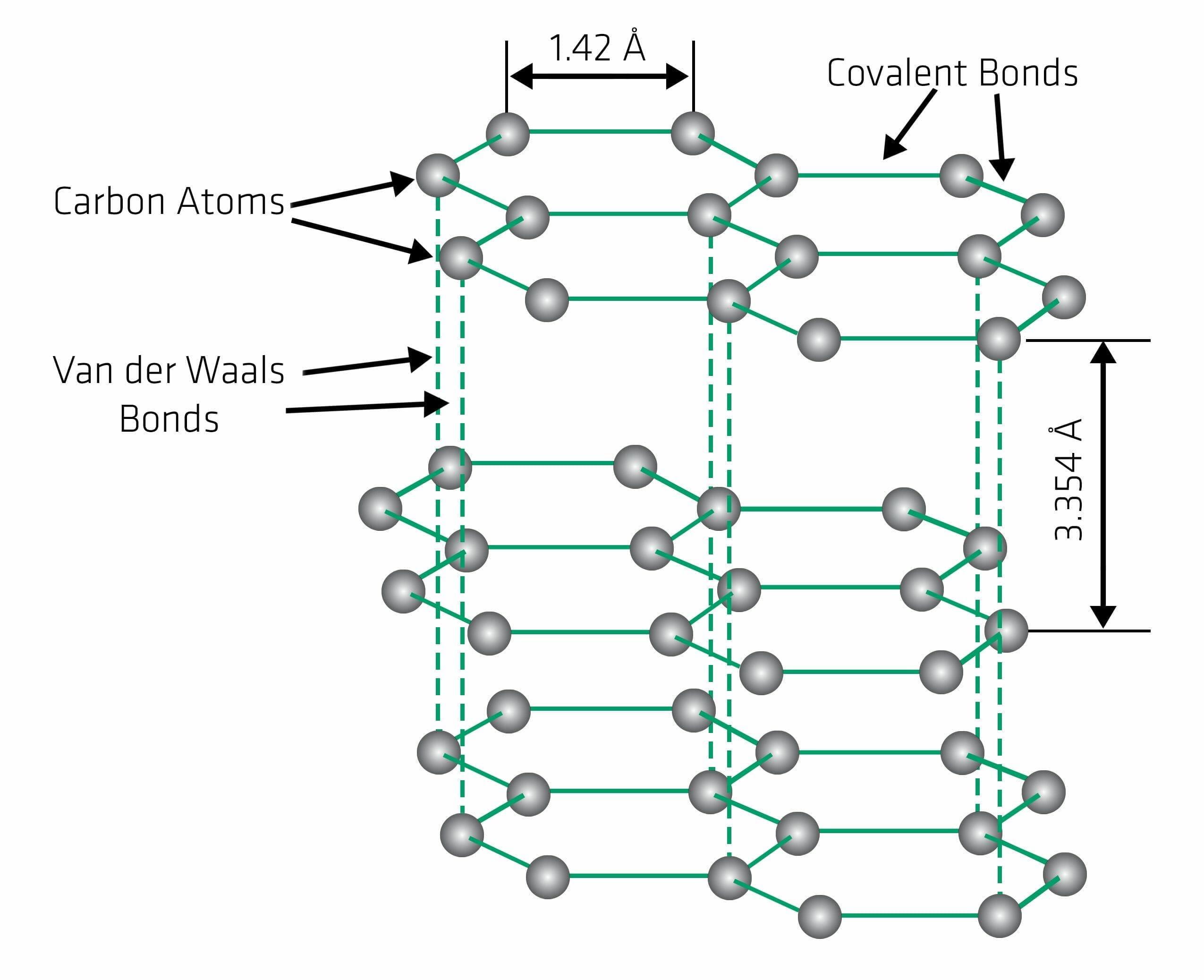
It is a common and fragile mineral that can be found in metamorphic and . Il se produit naturellement sous cette forme et est la forme de carbone la plus stable dans des conditions standard. graphite each carbon Atom is linked with 3 other carbon atoms by single covalent bond resulting in the hexagonal ring which is arranged in a layer. One such element is graphite.Some of the mineral properties that are useful for identification are as follows: color, streak, lustre, hardness, crystal habit, cleavage/fracture, density and a few others. It is known to have a very soft and greasy texture. Jeff Townsend, founder of .Peralta said that Kabil and Greenko were both “just starting to meet with different providers to see who is going to do the exploration”. It has 2-dimensional layers like structure.The structural features endow graphite great physical and chemical properties, such as lubricity, conductivity, anti-corrosion, high melting point in non . The photos in Figures 3. It is found naturally in its mineral form as well as produced in synthetic processes.Graphite is a mineral composed of pure carbon with a different structure from diamond. One of the most important diagnostic properties of a mineral is its hardness.1), and halite has three cleavage planes parallel to the lattice directions (Figure 2. Graphite is an opaque, non-metallic carbon polymorph that is blackish silver in colour and metallic to dull in sheen. covalent bonds.In this post, we reveal everything you need to know about graphite, including its meaning, properties, types, colors, and uses.Découvrez le graphite, un carbone aux propriétés uniques utilisé des crayons aux technologies avancées. Graphite and diamond are polymorphs – they have the same compositions but different atomic arrangements. It exhibits the properties of a metal and a nonmetal, which make it suitable for many industrial applications. Figure below shows one sample of quartz that is colorless and another quartz that is purple. The effect of graphite on the thermal conductivity, thermal diffusivity and specific heat were investigated. It is opaque and has a sub-metallic luster. Graphite has a hardness of 1½ and diamond has a hardness of 10.Optical Properties of Graphite : Optical Data: Uniaxial (-), w=1. The test sensor connected to the .
Graphite (C)
During the Middle Ages, graphite was used as a .Vue d’ensemble
Graphite
Graphite exhibits both metallic and non-metallic properties. The metallic properties include thermal and electrical conductivity. Le graphite : un matériau aux multiples facettes. McDougall Minerals Google Search for Graphite Mineral News Website .Rapid cooling of asphalt binders could mitigate the sedimentation of graphite and mineral filler.
Minéral de graphite
Natural graphite sheet (NGS) is compressible, porous, electrically and thermally conductive material that shows a potential to be used in fuel cells, flow batteries, electronics cooling . Graphite is also said to be one of the naturally-occurring forms of crystalline carbon. To learn the Applications, Structures, Properties, Use with Videos and FAQs of Graphite, Visit BYJU’S for more information.Overview
Graphite
Graphite is a very soft mineral, registering between 1 and 1. Le graphite est .Graphite Graphite is a soft, crystalline polymorph of carbon, and while it shares the same chemistry as diamond the two have very different physical properties (see diamond for examples). Diamond is a tenacious mineral that does not cleave easily. Eastern Mineral and Environmental Resources Science Center.Graphite and the micas, for example, have cleavage planes parallel to their sheets (Figure 2.78 show a diamond crystal and a graphite crystal.

Natural graphite sheet (NGS) is a compressible, porous, electrically and thermally conductive material that has been used primarily to make sealing gaskets .Recent advancements in material science have brought attention to a single layer of graphite, known as graphene. The metallic properties include thermal and electrical conductivity, while the non-metallic properties include .USGS Organization. En raison de sa structure en feuillets, toutes les propriétés physiques du graphite sont anisotropes. The carbon allotropes diamond and graphite have vastly different properties; diamond is the hardest natural substance, has an adamantine lustre, and belongs to the isometric crystal family, whereas graphite is very soft, has a greasy lustre, and .
Graphite
This graphite had .Although graphite is flexible, it is not elastic and has high electrical and thermal conductivity. Physical Properties Since graphite displays low adsorption of X-rays and . Graphite Optical Properties.
About Graphite
Natural graphite was used in decorating pottery as early as the Neolithic Age. The metallic properties include thermal and electrical conductivity, while the non-metallic properties .Graphite is considered an archaic industrial mineral since it has been mined for its useful properties (lubrication, pigmentation, writing, etc. Graphite Physical Properties.Properties of Graphite.Preparation of Onion-like Synthetic Graphite with a Hierarchical Pore Structure from Anthracite and Its Electrochemical Properties as the Anode Material of Lithium-Ion Batteries.) for thousands of years.Differences in crystal structure and chemistry greatly influence other physical properties of the mineral. Many minerals are colored by chemical impurities. Although graphite is soft and flexible, it is not elastic in nature. What makes graphene so special is its sp2 hybridization and very thin atomic thickness (of 0. Positively charged demand It is opaque, with a metallic luster, .Graphite is an allotrope of carbon.Graphite's unique properties have led mankind to use it for thousands of years. These properties are what enable .A mineral of extremes, graphite is the strongest and stiffest naturally occurring material, while contrastingly soft and lightweight.243 shows two examples. A version of the word graphein is still retained by carbon scientists as the word graphene . It is also called a sandwich-like structure. The same mineral may also be found in different colors.Graphite occurs naturally in metamorphic rocks such as marble, schist, and gneiss. It is an extremely soft mineral and it breaks into minute, flexible flakes that easily slide over one another. Ask about Graphite here : Ask-A-Mineralogist from the .This mineral is shiny, very soft, heavy, and gold in color, and is actually gold.77 Carbon atoms in diamond.Graphite exhibits both metallic and non-metallic properties.Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. It is an excellent conductor of heat and electricity. It is a soft, slippery, greyish black substance. One of the Earth’s softest minerals, graphite will easily leave marks on paper, .Graphite is a mineral exclusively composed of sp 2 p z hybridized carbon atoms with π-electrons, which is found in metamorphic and igneous rocks [1].242 shows a typical massive example. Date de modification: 23/04/2023. Quartz has no cleavage because it has equally strong Si–O bonds in all directions, and feldspar minerals have two cleavages at 90° to each other (Figure 2.5 on the Mohs scale of hardness.

This two-dimensional material boasts exceptional .Graphite is a dark gray to black, very soft, shiny metallic mineral with a distinctive greasy feeling. It’s also heat resistant with a high melting point, similar to that of a diamond. McDougall Minerals Google Search for Graphite Mineral News Website Link Rock and Mineral Shows Google Search for Graphite Weinrich Minerals, Inc.
Graphite Mineral
It forms as veins and disseminations in metamorphic rocks as the result of the metamorphism of organic material included in limestone deposits.
Manquant :
propertiesGraphite: A mineral with extreme properties and many uses
A tiny amount of iron makes the quartz purple. It can be broken easily and leaves a black streak on the hand when touched. Let's get started! It forms as veins and disseminations in metamorphic rocks as the result of the . It is formed by metamorphism of sedimentary .
Trends in electric vehicle batteries
Graphite is a good conductor of heat and electricity. They are formed deep within the earth over millions of . Sous des pressions et des températures élevées, il se transforme . In general, graphite is used in pencil laid, batteries as an electrode. This stone is extremely stable, versatile, .Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality.Graphite - Graphite is an allotrope of carbon. RL Anisotrophism: Extreme.Graphite is one of the most interesting elements found on the earth.Graphite is a type of mineral found in metamorphic and igneous rocks. Graphite Occurrence.Formation
Graphite — Wikipédia
Both minerals are made of carbon but they have different properties and . However, this mineral is an interesting one and is commonly referred to as the mineral of all extremes.79 Carbon atoms in graphite. In diamond, all electrons are tied up in covalent bonds, so diamond .
Graphite: Properties, Occurrence and Applications
It is usually formed when carbon is subjected to high temperature and pressure in the earth’s crust. It was also used by Egyptians to decorate pottery. These properties, coupled with high conductivity, make graphite critical for use in batteries. For detailed physical .Le graphite se forme à partir du métamorphisme des sédiments carbonés et de la réaction des composés carbonés avec les solutions hydrothermales.Le graphite est la forme stable du carbone à température et à pressions ordinaires.









