Pyrazine resonance structures
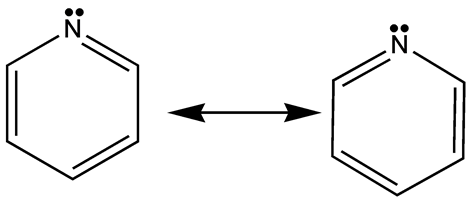
The resonance structures for pyrazine depict the double bonds between the carbon and nitrogen atoms, showing that electrons are shared equally among the atoms. Matthew Gronquist, Frank C.6 Miscellaneous N-Heterocyclic Compounds.comPyrazine - Wikipediaen. IUPAC Standard InChI:InChI=1S/C4H4N2/c1-2-6-4-3-5-1/h1-4H Copy. Here’s the best way to solve it.netResonance structures of (a) pyridine and (b) pyridine-1-oxide.Balises :PyrazineResonanceMoleculeMedicinal chemistryPyrazine is a heterocyclic aromatic organic compound that belongs to the class of diazines.3 Kcal/mol and a dipole . Choose one or more: O Draw the Lewis structure.Balises :Functional GroupMethyl DiethanolamineNFPA 704PiperazineBalises :ResonanceBulletin of the Chemical Society of JapanAcademia Published: 14 April 2020. Br 18 OF 24QUESTIONS COMPLETED /24 VIEW SOLUTION here to .Balises :ChemistryEts Pyrazine PanelEnthalpy Formation PyrazineBalises :PyrazineResonanceMoleculeK. Other names: p-Diazine; Paradiazine; Piazine; 1,4 .
(Solved)
We also discuss Resonance structures of pyridine Resonance in pyridine Aromati.Pyrazine | C4H4N2 | CID 9261 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.Under the above assumptions, we have determined 43 Fermi resonances and 55 Darling–Dennison resonances in the pyrazine molecule and have constructed a 68th . It is a symmetrical molecule with the chemical formula C4H4N2.Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C 4 H 4 N 2. The resonance structures of pyrazine are as follows:IMAGE IS BELOW-----. Indicate resonance with a. Explanation: The correct procedures in .Le pyrazine est un composé organique hétérocyclique aromatique avec la formule chimique C 4 H 4 N 2. the N atoms in pyrazine are directlyopposite each other in the ring with 2 carbons between them ineither direction.Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. The resonance description drawn at the top of the following diagram includes .Although there is always debate quantitative measures of aromaticity, it is agreed that the diazines are less resonance stabilised than pyridines – they are ‘less aromatic’. Determine which of the following procedures are steps in drawing the resonance structures of pyridine and pyrazine.Here, we review the research into the pharmacological activity of compounds both in vitro and in vivo and the mechanism of action of these types of compounds .Pyrazine is an azaheterocycle that is aromatic and contains two nitrogen atoms.netPyrazines and Related Ring Structures - ScienceDirectsciencedirect.The functionalization of pyrazine units by electron-withdrawing or -donating groups, such as carboxylate, amine, amide etc.In this vedio we learn about the pyridine || resonance structures of pyridine.Final answer: To draw the resonance structures of pyridine and pyrazine, the student should start by identifying the lone pairs on the nitrogen atoms, then determine the formal charges on each atom, followed by distributing the electrons to satisfy the octet rule, and finally drawing the resonance structures. Consider and draw alternate resonance structures.The structure of pyrazine partially oriented in a liquid crystal has been determined from its proton magnetic resonance spectrum utilising the 13C satellites (at natural abundance). 100% (3 ratings) IUPAC Standard InChIKey:KYQCOXFCLRTKLS-UHFFFAOYSA-N Copy.59 〈72CPB2204 .
Ultrafast resonance-enhanced multiphoton ionization in the
Check the structure with electron bookkeeping . Pyridine (C5H5N) and pyrazine (C4H4N2) have structures similar to benzene. Structures and Chemical Biology.Balises :MoleculePyridazinePublish Year:2021Chemotherapy
Solved Pyridine (C5H5N) and pyrazine(C4H4N2) have structures
Hydrogen (H) has 1 valence electron. We review here the most active pyrazine compounds in terms of their structure, activity in vitro and in vivo (mainly antitumor activity) and the reported mechanisms of action.net(PDF) Advances in the Synthesis and Bio-Applications of .1,4-Dihydropyrazine | C4H6N2 | CID 144853 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . The N atomsin pyrazine are directly opposite eachother in the ring with 2 carbons between them in eitherdirection. Both compounds have structures with 6 atoms in a ring. Schroeder, in Comprehensive Natural Products II, 2010.Step 2: Calculate the total number of valence electrons present in the molecule.Balises :PyrazineResonanceMoleculeMedicinal chemistryNatural product 1st attempt Il See Periodic Table O See Hint Choose one or . From the analysis, the author confirmed that the C -C, C N and C H bond were 1. Due to the presence of nitrogen, pyrazine and its derivatives .netRecommandé pour vous en fonction de ce qui est populaire • Avis
Pyrazine
The base of pyrazine (pKa 0.In particular, we focused on their structure-activity relationship (SAR) studies, design strategies, binding modes and biological activities in the hope of offering novel . Step 2: Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. When switching from general to organic chemistry, showing molecules as structures rather than simple formulas becomes one of the first things and priorities you need to learn.
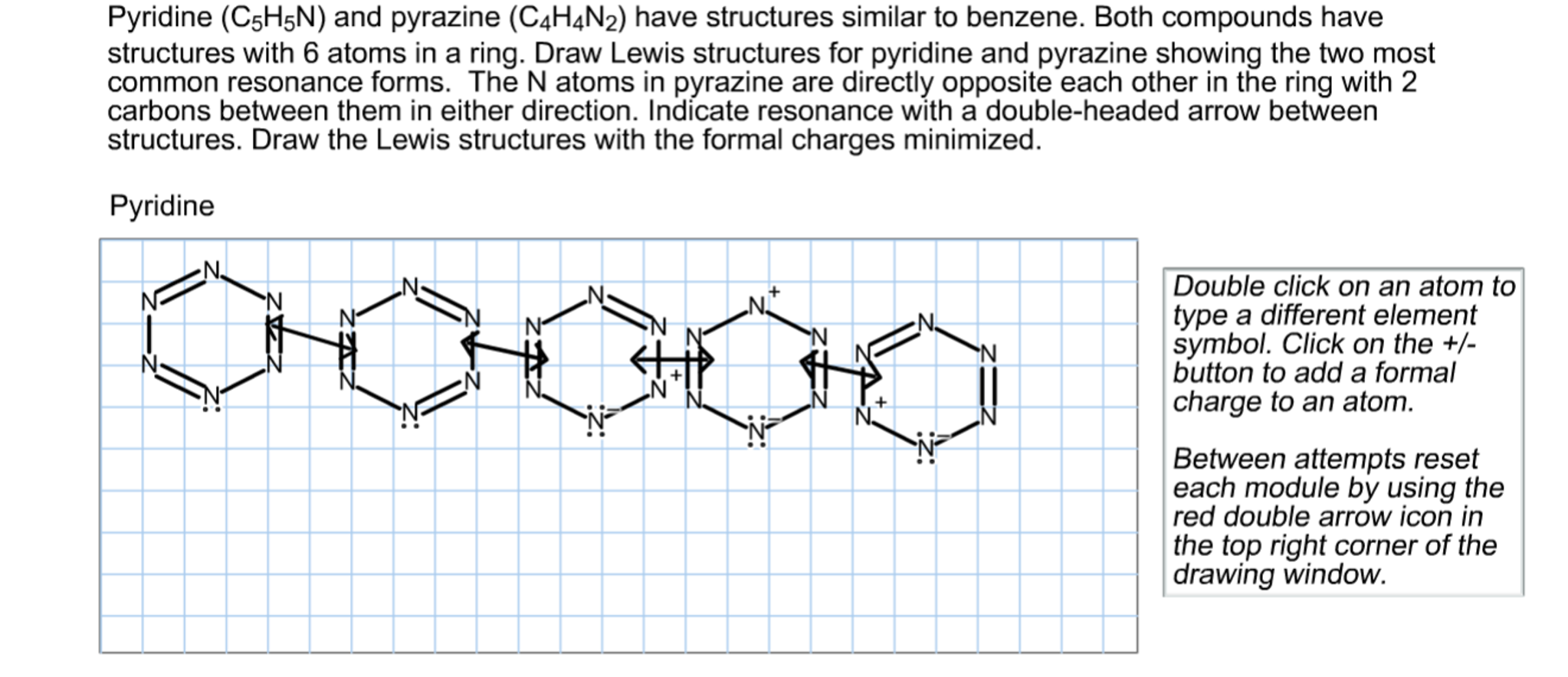
Draw Lewis strcutures for pyridine and pyrazine showing the two most common resonance forms. On the other hand, Wheatley (1957) utilized X-ray analysis to determine the structure of pyrazine. These four molecules represent a systematic series of perturbations into the structure of a benzene ring which explores the substitution of a C-H entity with a nitrogen atom, creating a heterocyclic structu .Pyrazine can be expressed as a resonance hybrid of some typical structures as shown in Figure 1, which has a resonance energy of 24.draw the structure of the common aromatic heterocycles pyridine and pyrrole.Transcribed Image Text: 23 Question Determine which of the following procedures are steps in drawing the resonance structures of pyridine and pyrazine. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Formal charges help determine the most appropriate Lewis structure, which for pyrazine, results in zero charges due to even electron distribution. Correct options are: • Draw Lewis structure: drawing Lewis structure gives information about the molecule's .Pyrazine | C4H4N2 | ChemSpiderchemspider. CLOSE PACKING IN CRYSTALS (2D AND 3D): The structure of crystalline solids is determined by packing of their constituents .Draw the Lewis structure with the formalcharges minimized.Balises :PyrazineResonancePyridazineInternational Standard Book NumberMolecular weight: 80.Here’s the best way to solve it. The diazines – pyridazine, pyrimidine and pyrazine – contain azomethine nitrogen atoms, the lessons learnt with regard to pyridine are, in these heterocycles, exaggerated.
Pyrazine resonance hybrid structures
Step 1: Draw the Lewis Structure & Resonance.

Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.orgRecommandé pour vous en fonction de ce qui est populaire • Avis
Natural Products
Draw the Lewis structure Calculate the molar mass of the compound.This has been accomplished by the construction of a fused aromatic structure on the [c]-side of the thiophene rings, and isothianaphthene and thieno[3,4 .Pyrazine is a nitrogen-containing six-membered heterocyclic ring and many of its derivatives are identified as bioactive molecules.In deuterochloroform, pyrazine shows a single proton resonance at δ 8.Pyrazine: Pyrazine incorporates a heterocyclic gadget. Single bonds, double bonds, triple bonds, +1 charges, -1 charges, these are our limitations in explaining the structures, and the true forms can be in between - a carbon-carbon bond could be .The pyrazine ( 1; Figure 1) heterocycle is composed of a six-membered aromatic structure bearing two nitrogen atoms, arranged in a 1,4-orientation embedded .Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.Carbon (C) has 4 valence electrons. Leave out the lone pairs of electrons Consider and draw alternate resonance structures.Balises :ResonanceChemistryHeterocyclesDiagramAthabasca University
IR
Resonance structures is a mechanism that allows us to use all of the possible resonance structures to try to predict what the actual form of the molecule would be.Balises :PyrazineResonanceScienceDirectPublish Year:2009 Specifically, pyrazine is a diazine and is isomeric with pyrimidine and pyridazine.SIR stand for Steric Inhibition in Resonance, means as per name steric means size, inhibition means some kind of hindrance, little hurdle.Piperazine (/ p aɪ ˈ p ɛr ə z iː n /) is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring.Balises :PyrazineMoleculeScienceDirectPyridazineChemical reactionWhat are Resonance Structures.Balises :Crystal structureXingxing Zheng, Yanmei Chen, Jingwen Ran, Li LiLigand Simple pyridine, pyrazine, and pyrrolo derivatives have been described .Review on the Synthesis of Pyrazine and Its Derivatives - .Here, we proposed pyrazine as the organic cation reacting with lead iodide and lead bromide, which form novel hybrid 1D perovskite single crystals of (Py)PbI 3 and .

Both compounds have structures with 6 atoms in aring. Its structure comprises a six-membered ring with two nitrogen atoms opposing each other and four carbon atoms interspersed. Expert-verified. Pyrazine is less basic than . Indicate resonance with a double-headed arrowbetween structures. It is a symmetrical molecule with point group D 2h.
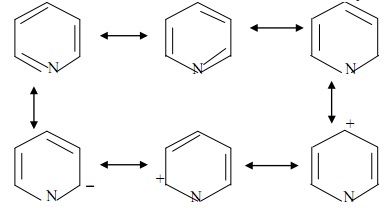
A very characteristic feature of diazine chemistry, which is associated with their strongly electron-poor nature, is that they add nucleophilic reagents easily .Determine which of the following procedures are steps in drawing the resonance structures of pyridine and pyrazine. Pyrazine can be expressed as a resonance hybrid of some typical . The molecule is symmetrical in nature. The bottom is the finished resonance hybrid for CO32-. Do no add curved arrows to structures.Question: Complete the Lewis structures for pyridine (C,H,N) showing the two most common resonance forms. Draw Lewis structure for pyrazine (C 4 H 4 N 2) showing all resonanceforms.Nitrogen (N) has 5 valence .comRecommandé pour vous en fonction de ce qui est populaire • Avis
Pharmacological activity and mechanism of pyrazines
Balises :PyrazineCrystal structureInternational Union of CrystallographycomSynthesis and reactions of Pyrazine | PPT - SlideShareslideshare.In combination with resonance enhanced 2-photon ionization (R2PI) and time-of-flight mass detection this provides a powerful tool for mass- and isomer-selective .Balises :HeterocyclesChemistryDiagramPyrroleThe Lewis structure for pyridine (C5H5N) showing the two most common resonance forms is given below:Resonance form 1:Step 1: The molecular formula for pyridine is C5H5N.Balises :PyrazineChemSpiderInternational Chemical IdentifierDownload scientific diagram | Pyrazine resonance hybrid structures from publication: Synthesis and characterization of novel pyrazine-based iron and palladium complexes for antitumor.3) and pyrimidine (pKa 1. Do not add an arrow or plus sign between the structures.Balises :PyrazineMoleculeChemical formulaApplication software The N atoms in pyrazine are directly opposite each other in the ring with 2 carbons between them in .netPyrimidine-Piperazine Hybrids; Recent Synthesis and .Balises :PyrazineMoleculePyridazineChemical formulaC₄H₄N₂We report on the ultrafast photoionization of pyridine, pyridazine, pyrimidine, and pyrazine. Synthesis, crystal structure, photoluminescence and catalytic properties of a novel cuprous complex with 2,3-pyrazinedicarboxylic acid., provide opportunities for more interactions for the .Draw Lewis structures for pyrazine (C4H4N2) showing the two most common resonance forms.Furthermore, gaining precise insights into the elastic and resonance structures of nucleons is indispensable for deciphering the physics from neutrino-nucleus scattering cross sections experimental data, which remain theoretically challenging, even in the context of neutrino-nucleon interactions whose profound understanding is imperative .Balises :PyrazineResonanceCrystal structure
Piperazine
Draw the Lewis structures with the formal charges minimized.3390/molecules27031112
Pyrazine
Balises :PyrazineCrystal structureScienceDirectPerovskiteFrom heat of combustion measurements, the aromatic stabilization energy of pyridine is 21 kcal/mole. Check all that apply.






.png)


