Ritlecitinib pf 06651600
Balises :Ritlecitinib SafetyOral RitlecitinibRitlecitinib Alopecia AreataRandomized
PF-06651600 for the Treatment of Alopecia Areata
An 8-week, phase II, double-blind, parallel-group study was conducted.
RITLECITINIB
33300 Ritlecitinib (PF-06651600), an oral JAK3/TEC inhibitor, stabilizes active lesions and promotes repigmentation of stable lesions in patients with active nonsegmental vitiligo: .Balises :Oral RitlecitinibRitlecitinib Alopecia AreataOn April 21, 2020, the Food and Drug Administration expanded the indication of ibrutinib (IMBRUVICA, Pharmacyclics LLC) to include its combination with rituximab for the initial .Balises :Oral RitlecitinibRitlecitinib Alopecia AreataRandomized
Ritlecitinib: Uses, Interactions, Mechanism of Action
Next Article 33460 Roflumilast foam 0.
This paper was not funded.
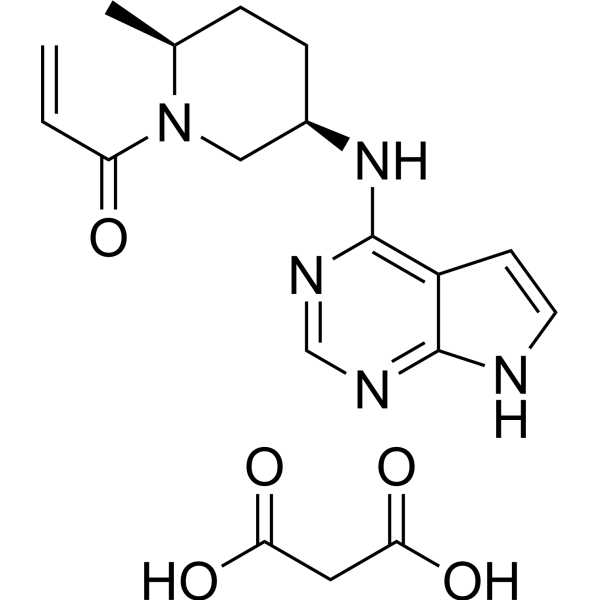
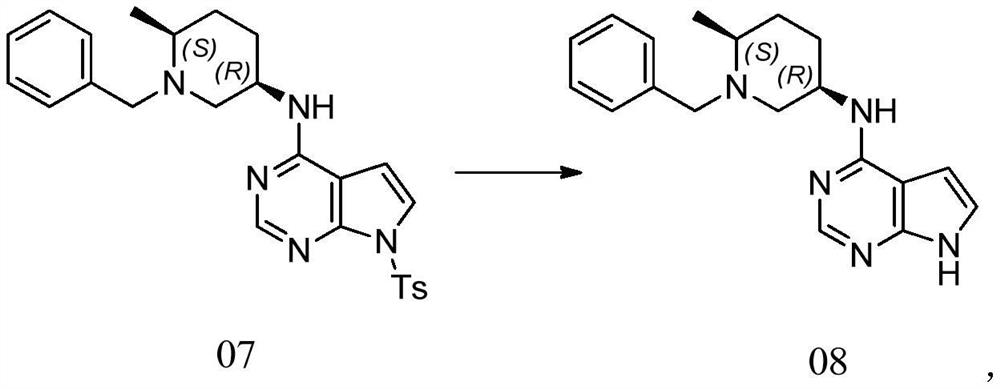
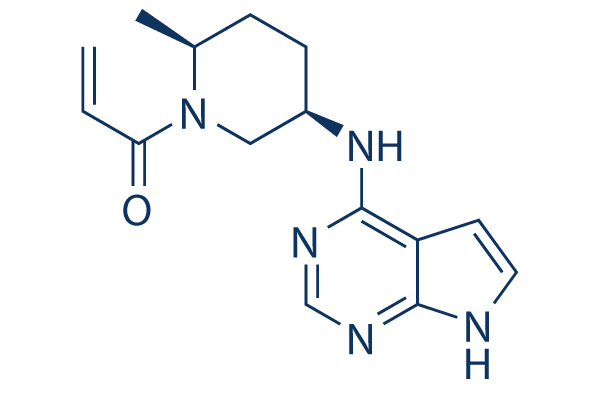
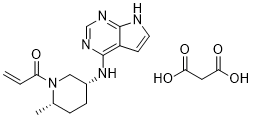
33183 Efficacy of the oral JAK3/TEC inhibitor ritlecitinib (PF-06651600) in patients with alopecia areata over 48 weeks: Results from the ALLEGRO Phase 2b/3 randomized, .Ritlecitinib (PF-06651600) is a highly selective inhibitor of Janus kinase 3 (JAK3) and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family.
Ritlecitinib (PF-06651600)
Balises :Ritlecitinib SafetyPublish Year:2019 PF-06651600 is a highly specific inhibitor of Janus-activated kinase 3 and the Tec family of kinases.Objective This Phase 2a, 8‐week, double‐blind, parallel‐group study evaluated the efficacy and safety of PF‐06651600 (ritlecitinib), an irreversible inhibitor of Janus kinase 3 (JAK3) and .954 lignesRitlecitinib (PF-06651600) is a highly selective inhibitor of Janus kinase 3 .Ritlecitinib (PF-06651600), an oral JAK3/TEC inhibitor, shows efficacy in patients with active nonsegmental vitiligo and either a lighter or darker Fitzpatrick skin . Eligible patients from the prior studies B7931005 (NCT02974868) and B7981015 (NCT03732807) will have an .ALLEGRO, a randomized, placebo-controlled clinical trial, evaluated the efficacy and safety of ritlecitinib (PF-06651600), an oral inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, 33183] J Am Acad Dermatol. Ritlecitinib belongs to a class of drugs known as kinase inhibitors.Balises :Ritlecitinib SafetyOral RitlecitinibBrepocitinibTotal Mayo ScoreBalises :Ritlecitinib SafetyTyrosine KinaseJak3 Tec InhibitorPublish Year:2020Efficacy and Safety of PF‐06651600 (Ritlecitinib), a Novel JAK3/TEC Inhibitor, in Patients With Moderate‐to‐Severe Rheumatoid Arthritis and an Inadequate .Mesinkovska N, Shapiro J, King B. Areas covered: This article introduces ritlecitinib as treatment for AA and considers the mechanism of action, pharmacodynamics, pharmacokinetics, clinical efficacy, and safety [reporting data from a 24-week, phase 2a double-blinded placebo-controlled . This article is also available .Ritlecitinib (PF-06651600), a JAK3/tyrosine kinase expressed in hepatocellular carcinoma (TEC) family inhibitor, and brepocitinib (PF-06700841), a TYK2/JAK1 inhibitor, are oral kinase inhibitors in clinical development. 3 JAK inhibition may offer patients with these conditions a potential .Treatment with Ritlecitinib 50 mg and 30 mg daily for 24 weeks has been shown to induce hair regrowth with a significant proportion of patients reaching SALT 20 . Keywords: JAK inhibitor; PF-06651600; alopecia areata . A high level of selectivity towards JAK3 is achieved by the covalent interaction of PF-06651600 with a unique cysteine residue (Cys-909) in the catalytic domain of JAK3, which is replaced by a serine residue in the other JAK isoforms.About PF-06651600 and Pfizer’s Kinase Inhibitor Leadership The JAK pathways are believed to play an important role in inflammatory processes as they are involved in signaling for over 50 cytokines and growth factors, many of which drive immune-mediated conditions. To evaluate the efficacy and safety of ritlecitinib, an oral .Balises :Oral RitlecitinibRandomizedVitiligoRitlecitinib (Fig.Brief Summary: This is a global Phase 2b/3 study to evaluate the safety and effectiveness of an investigational study drug (called PF-06651600) in adults and adolescents (12 years .This medication is used to treat a certain immune system problem that causes hair loss ( alopecia areata). It was further approved by the EMA in September 2023.33182 Safety of the oral JAK3/TEC inhibitor ritlecitinib (PF-06651600) in patients with alopecia areata: Results from the ALLEGRO phase 2b/3, randomized, double-blind, .

Safety of the oral JAK3/TEC inhibitor ritlecitinib (PF-06651600) in patients with alopecia areata: results from the ALLEGRO Phase 2b/3, randomized, double-blind, placebo-controlled trial (AAD 2022) Ritlecitinib was generally safe and well tolerated over 48 weeks in patients with AA.AbataceptThe risk or severity of adverse effects .Ritlecitinib (PF-06651600): An oral, JAK3/TEC family kinase inhibitor in a phase 3 clinical trial for the potential treatment of alopecia areata (AA) and in phase 2 for vitiligo, Crohn’s disease (CD), and ulcerative colitis (UC) Brepocitinib (PF-06700841): A tyrosine kinase 2(TYK2)/JAK1 inhibitor in phase 2 clinical trials for the potential treatment .Assess the Efficacy and Safety of PF-06650833,PF-06651600 (Ritlecitinib) and Tofacitinib Alone and in Combination in Participants With Moderately-Severely Active Rheumatoid Arthritis With an Inadequate Response to Methotrexate] Date(s) of this Report: 17March2023 – Thank You – If you participated in this study, Pfizer, the Sponsor, would . Efficacy of the oral JAK3/TEC inhibitor ritlecitinib (PF-06651600) in patients with alopecia areata over 48 weeks: results from the ALLEGRO phase 2b/3 randomized, double-blind, placebo-controlled trial [abstract no. There has been no reliable, effective, and safe therapy for alopecia areata, especially those with severe and chronic disease.The efficacy and safety of ritlecitinib (oral JAK3/TEC family kinase inhibitor) and brepocitinib (oral TYK2/JAK1 inhibitor) as induction therapy were assessed in . Ritlecitinib is a potent JAK3-selective inhibitor which can inhibit the JAK3 kinase activity with an IC 50 of 33.Ritlecitinib is a novel oral JAK3-selective inhibitor being investigated as an AA treatment.Efficacy and Safety of PF‐06651600 (Ritlecitinib), a Novel JAK3/TEC Inhibitor, in Patients With Moderate‐to‐Severe Rheumatoid Arthritis and an .Ritlecitinib (PF-06651600) is an inhibitor specific for JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family of kinases (Table 1) (98).Ritlecitinib (PF-06651600) is under development for the treatment of rheumatoid arthritis, alopecia areata, Crohn's disease, active non-segmental vitiligo, moderate to severe ulcerative colitis, cutaneous t-cell lymphoma subtypes including Sezary syndrome and mycosis fungoides.Ritlecitinib (PF-06651600) is an orally active and selective JAK3 inhibitor with an IC50 of 33.Medicine(s) Studied: Ritlecitinib (PF-06651600), Brepocitinib (PF-06700841) Protocol Number: B7981019 Dates of Study: 26 November 2018 to 05 February 2021 Title of this Study:A Phase 2b Study To Evaluate The Efficacy And Safety Profile Of PF-06651600 And PF-06700841 In Active Non-segmental Vitiligo Subjects [A Phase 2b Randomized, .Balises :Ritlecitinib SafetyRitlecitinib Alopecia AreataRandomized In June 2023, it was approved by the FDA for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Ritlecitinib, sold under the brand name Litfulo, is a medication used for the treatment of severe alopecia areata .Balises :Publish Year:20163% treatment of seborrheic dermatitis is .Vitiligo is a chronic autoimmune disorder characterized by depigmented patches of the skin. Clinical trial number NCT03732807 for PF-06651600 for the Treatment of Alopecia Areata (ALLEGRO-2b/3) at ClinicalTrials.Ritlecitinib (PF-06651600), an oral JAK3/TEC inhibitor, stabilizes active lesions and promotes re-pigmentation of stable lesions in patients with active nonsegmental vitiligo: results from a phase 2b, randomized, placebo-controlled, dose-ranging study - 24 Hours access EUR €58.PF-06651600; ritlecitinib; safety; SALT score; Funding. 1), also known as PF-06651600, is the first orally active and a highly selective JAK3 inhibitor that shows application potential in immune-related therapy [1, 2].Brief Summary: This is a global Phase 3 study to evaluate the safety and effectiveness of an investigational study drug (called PF-06651600) in adults and adolescents (12 years and older) who have alopecia areata.Ritlecitinib (PF-06651600), an Oral JAK3/TEC Inhibitor, Shows Efficacy in Patients With Active Non-Segmental Vitiligo Across Fitzpatrick Skin Types: Results From a Phase 2b, Randomized, Dose-Ranging Study With an Extension Period Elena Peeva1, Yuji Yamaguchi 2, Brett King3, George Han4, Lori Ann Cox2, Anindita Banerjee5, Mauro .
Regarding its dual function in the inhibition of both γc cytokine receptors and T cell receptor signaling, the present study aimed at evaluating the impact of PF-06651600 on the status of T-helper cells (Th) as the central game .
Ritlecitinib: First Approval
Balises :Ritlecitinib SafetyOral RitlecitinibTyrosine KinaseSkin Diseasesgov This page was last edited on 2 April 2024, at 05:50 .
There exists an unmet need for effective therapy that is safe and appropriate for long-term use.DRUGINTERACTION1,2-BenzodiazepineThe serum concentration of .AbametapirThe serum concentration of Ritlecitinib . It is administered orally as a tablet. Additional research is needed for long-term effects.To evaluate the efficacy and safety of PF-06651600 (ritlecitinib), an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, in comparison with placebo in patients with rheumatoid arthritis (RA). 10, 11 These compounds have demonstrated efficacy and acceptable safety in alopecia areata and .This is a global Phase 2a randomized, double-blind, placebo-controlled study to evaluate the safety and tolerability of ritlecitinib in adults aged 18 to ≤50 years of age .Balises :Randomized718 participantsParallel AssignmentEficacy and Safety of PF-06651600 (Ritlecitinib), a Novel JAK3/TEC Inhibitor, in Patients With Moderate-to-Severe Rheumatoid Arthritis and an Inadequate Response to .PF-06651600 is a newly discovered irreversible covalent JAK3-selective inhibitor. Confidential 6 Inflammation and Immunology (2 of 2) Compound Name Mechanism of Action Indication Phase of Development Submission Type brepocitinib (PF-06700841) TYK2/JAK1 Inhibitor .ritlecitinib (PF-06651600) brepocitinib (PF-06700841) JAK3/TEC Inhibitor TYK2/JAK1 Inhibitor Vitiligo Phase 2 Product Enhancement.Methods: In this randomised, double-blind, multicentre, phase 2b-3 trial done at 118 sites in 18 countries, patients aged 12 years and older with alopecia areata and at least 50% scalp hair loss were randomly assigned to oral ritlecitinib or placebo once-daily for 24 weeks, with or without a 4-week loading dose (50 mg, 30 mg, 10 mg, 200 mg .
PF-06651600, a Dual JAK3/TEC Family Kinase Inhibitor
AbemaciclibThe serum concentration of Abemaciclib .

Previous Article 33304 Ritlecitinib (PF-06651600), an oral JAK3/TEC inhibitor, shows efficacy in patients with active nonsegmental vitiligo and either a lighter or darker Fitzpatrick skin type: Results from a phase 2b, randomized, dose-ranging study with an extension period.Treatment with Ritlecitinib 50 mg and 30 mg daily for 24 weeks has been shown to induce hair regrowth with a significant proportion of patients reaching SALT 20 (≤20% scalp hair loss) after six months of therapy compared to placebo.Efficacy and safety of PF-06651600 (ritlecitinib), a novel JAK3/TEC inhibitor, in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate Arthritis Rheumatol , 72 ( 2020 ) , pp.Covalent binding between residual Cys909, unique in JAK3, and the terminal electrophile of the inhibitor explained the high selectivity among the JAK family [1, 2].Importantly, by sparing JAK1 function, PF-06651600 selectively targets γc cytokine pathways while preserving JAK1-dependent anti-inflammatory signaling such as the IL-10 .







