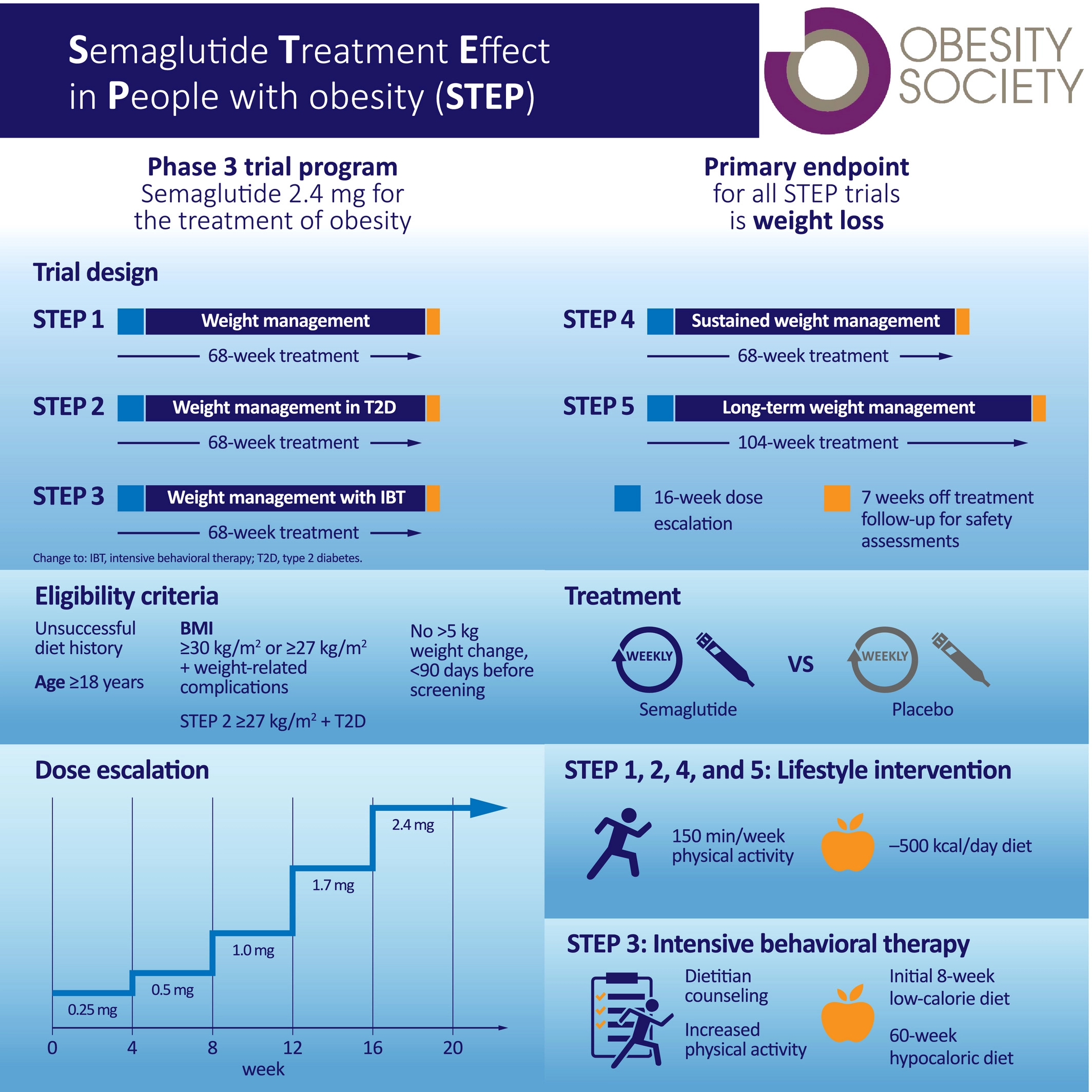
Semaglutide phase 3 trial
2%, respectively; P < .Substance: semaglutide Universal Trial Number: U1111-1215-7560 EUdraCT Number: 2018-002431-18 Trial phase:3a.Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial - The . Differences exist between Asian and non-Asian populations in terms of body composition and definitions of obesity.Methods: Across five phase 3 trials (NCT03548935, WM; NCT03552757, WM in type 2 diabetes; NCT03611582, WM with intensive behavioral therapy; NCT03548987, sustained WM; and NCT03693430, long-term WM), ~5,000 participants are being randomly assigned to receive semaglutide 2. MAESTRO-NAFLD-1 was a 52-week randomized, double-blind, placebo-controlled phase 3 trial evaluating .Participants will either get semaglutide or placebo (a dummy medicine which does not contain any study medicine) - which treatment participants get is decided by an equal chance. In a phase 2 dose-finding trial, oral semaglutide 40 mg taken once per day resulted in bodyweight reductions of 5·7 kg over 26 weeks in people with type 2 diabetes, with or without overweight or Methods: In an open-label, 40-week, phase 3 trial, we randomly assigned 1879 patients, in a 1:1:1:1 ratio, to receive tirzepatide at a dose of 5 mg, 10 mg, or 15 mg or semaglutide at a dose of 1 mg. In the Semaglutide Treatment Effect in People with obesity (STEP) 6 trial, we assessed the effect of . In the phase 2 trial of semaglutide in adults with obesity, a 0.govAn Oral Version of Wegovy Appears As Effective as the .
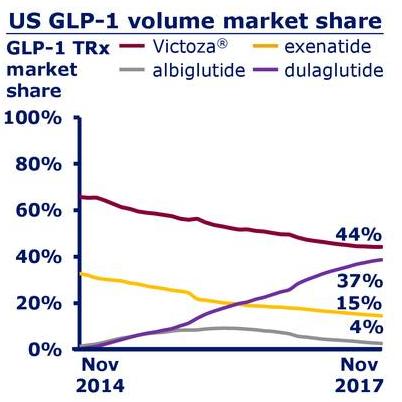
Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide
“Alzheimer’s disease has been an area of extensive research in the past .
Semaglutide once a week in adults with overweight or obesity
Novo Nordisk will soon start enrolling patients into phase 3 trials to test its oral diabetes drug Rybelsus (semaglutide) for treating .Nonalcoholic steatohepatitis (NASH) is a progressive liver disease with no approved treatment.4 mg Once Weekly on Function and Symptoms in Subjects With Obesity-related Heart Failure With Preserved Ejection Fraction: Actual Study Start Date : March 16, 2021Oral semaglutide 50mg achieved superior weight loss in adults with obesity or overweight with 1 or more comorbidities compared with placebo, according to results from the phase 3a OASIS 1 trial . The STEP 5 trial assessed the efficacy and safety of once-weekly subcutaneous semaglutide 2. On December 16, 2020, Novo Nordisk announced it would begin development of semaglutide in people with early Alzheimer’s disease (see press release).Sponsor staff involved in the clinical trial is masked according to company standard procedures. The global phase 3 Semaglutide Treatment Ef-fect in People with Obesity (STEP) program aims to .
Resmetirom for nonalcoholic fatty liver disease: a randomized
The SURMOUNT-3 is a company-funded, double-blind, placebo-controlled, randomized clinical trial, aiming to determine the effects of tirzepatide, a dual glucose .These findings suggest that both formulations of semaglutide are seemingly effective HbA1c and weight-lowering agents in people with T2D.The efficacy and safety of once-weekly tirzepatide as compared with semaglutide, a selective GLP-1 receptor agonist, are unknown.3% in the group receiving 1.8% for liraglutide 3.In an open-label, 40-week, phase 3 trial, we randomly assigned 1879 patients, in a 1:1:1:1 ratio, to receive tirzepatide at a dose of 5 mg, 10 mg, or 15 mg or semaglutide at a dose of 1.Objective: To investigate the dose-response relationship of semaglutide versus placebo and open-label liraglutide in terms of glycemic control in patients with type 2 diabetes.
Bagsværd, Denmark, 24 March 2023 – Novo Nordisk today announced headline results from the PIONEER PLUS trial, a phase 3b, 68-week, efficacy and safety trial with once .Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial.8 cm in waist circumference change, 4 mm Hg in systolic blood pressure change, and 3.This data supports and further validates the evaluation of semaglutide in an older population with early Alzheimer’s Disease in the Phase III EVOKE trials that aim to . In the following, Novo Nordisk A/S and its affiliates will be stated as “Novo .A higher proportion of participants in the semaglutide vs placebo group achieved weight losses of at least 10% or 15% (75. Objective: To compare the efficacy and adverse event profiles of once-weekly subcutaneous semaglutide, 2.About the OASIS clinical trial programme OASIS is a phase 3 clinical development programme with once-daily oral semaglutide 25 mg and 50 mg in obesity. In the following, Novo Nordisk A/S and its affiliates will .0%, which was .Oral semaglutide is coformulated in a tablet with the absorption enhancer sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate.4 mg once weekly subcutaneously versus placebo. The product is .Across five phase 3 trials NCT03548935, WM; NCT03552757, WM in type 2 diabetes; NCT03611582, WM with intensive .Background: Semaglutide 2·4 mg once weekly has been investigated for weight management in global populations. Abstract Importance Glucagon-like peptide-1 (GLP-1) receptor agonists are effective therapies for the treatment of type 2 diabetes and are all currently available as an injection.Importance: Phase 3 trials have not compared semaglutide and liraglutide, glucagon-like peptide-1 analogues available for weight management.4‐mg dose daily was well tolerated, and patients experienced a mean WL at week 52 from baseline of −13.4 mg in the STEP-3 trial produced a mean weight loss of 16.4 mg versus placebo (both plus behavioral intervention) .The preliminary estimate of k = 95 kcal/day per kilogram weight loss derived using data from SGLT2 inhibition [] was reasonably close to the best fit value of k = 83 . Go to Top of Page Study Description Study Design Arms and Interventions Outcome Measures .The STEP 5 trial assessed the efficacy and safety of once-weekly subcutaneous semaglutide 2.7% at baseline to 7.comRecommandé pour vous en fonction de ce qui est populaire • Avis
Semaglutide 2·4 mg once a week in adults with overweight or

The global clinical phase 3 programme currently consists of four trials, having enrolled approximately 1,300 adults with obesity or overweight with one or more comorbidities.Phase 3 studies are warranted to assess longer-term and clinical outcomes, as well as safety.This double-blind, double-dummy, phase 3, superiority study enrolled adults with a body-mass index of at least 27 kg/m 2 and glycated haemoglobin 7–10% (53–86 mmol/mol) who had been diagnosed with type 2 diabetes at least 180 days before screening.4 mg versus placebo (both plus behavioral intervention) for long-term treatment of adults with obesity, or overweight with at least one weight-related comorbidity, without diabetes.Auteur : Melanie Davies, Louise Færch, Ole K Jeppesen, Arash Pakseresht, Sue D Pedersen, Leigh Perreault, Jul. Treatment was discontinued owing to these . Participants will have 17 clinic visits and 1 phone call with the study doctor.Drug: Semaglutide Drug: Placebo: Phase 3: Study Design.The Phase 3a trial will enroll about 3,700 people in the early stages if Alzheimer’s disease, and will evaluate the efficacy and safety of 14 mg of oral semaglutide, compared with a placebo, taken once daily.At week 104, among patients receiving semaglutide, the mean glycated hemoglobin level decreased from 8.The SCALE Diabetes trial of once a day liraglutide 3·0 mg as an adjunct to lifestyle intervention in patients with overweight or obesity, and type 2 diabetes (n=846) reported a reduction in bodyweight of 5·4% from baseline.0 mg (both with diet and physical activity), in .2−4 In this randomised, double-blind, placebo-controlled, phase 3, superiority trial, conducted in nine countries in Asia, Europe, and North America, 334 participants were randomly assigned .In a phase 2 trial, once-daily subcutaneous semaglutide, 0.Nature Biotechnology 39 , 127 ( 2021) Cite this article.Efficacy and safety of once-daily oral semaglutide 25 mg and .8 mg once weekly), significantly increased weight loss vs liraglutide, 3.
Efficacy and safety of once-weekly semaglutide 2·0 mg versus
8% compared with −7.
News Details
6% in the group receiving 0. The study will last for up to 173 weeks (about 3 years and 4 months).Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B .

The OASIS 1 trial by Filip K Knop and colleagues1 demonstrates the greatest magnitude of weight loss to date among oral anti-obesity medications.
Methods: This was a phase II, open-label, proof-of-concept trial in which patients with NASH (F2-F3 on biopsy, or MRI-proton density fat fraction [MRI-PDFF] ≥10% and liver stiffness by transient elastography ≥7 kPa) were randomised to 24 weeks' treatment with semaglutide 2.These included ten phase 3 trials in the global PIONEER programme, which investigated oral semaglutide at doses of 7 mg and 14 mg daily.4 mg or corresponding placebo.who were participants in a phase 2 trial,12-14 findings that supported further investigation.verywellhealth.Oral semaglutide received FDA approval in September 2019 for use as adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes ( 1 ).
Oral semaglutide: an OASIS from injectables
Interestingly, there are . Patients (n = 415) were randomized to receive a subcutaneous injection of .This randomised, double-blind, placebo-controlled, phase 3, superiority trial enrolled adults with a BMI of at least 30 kg/m 2, or at least 27 kg/m 2 with bodyweight .
Diabetes drug on trial for Alzheimer’s
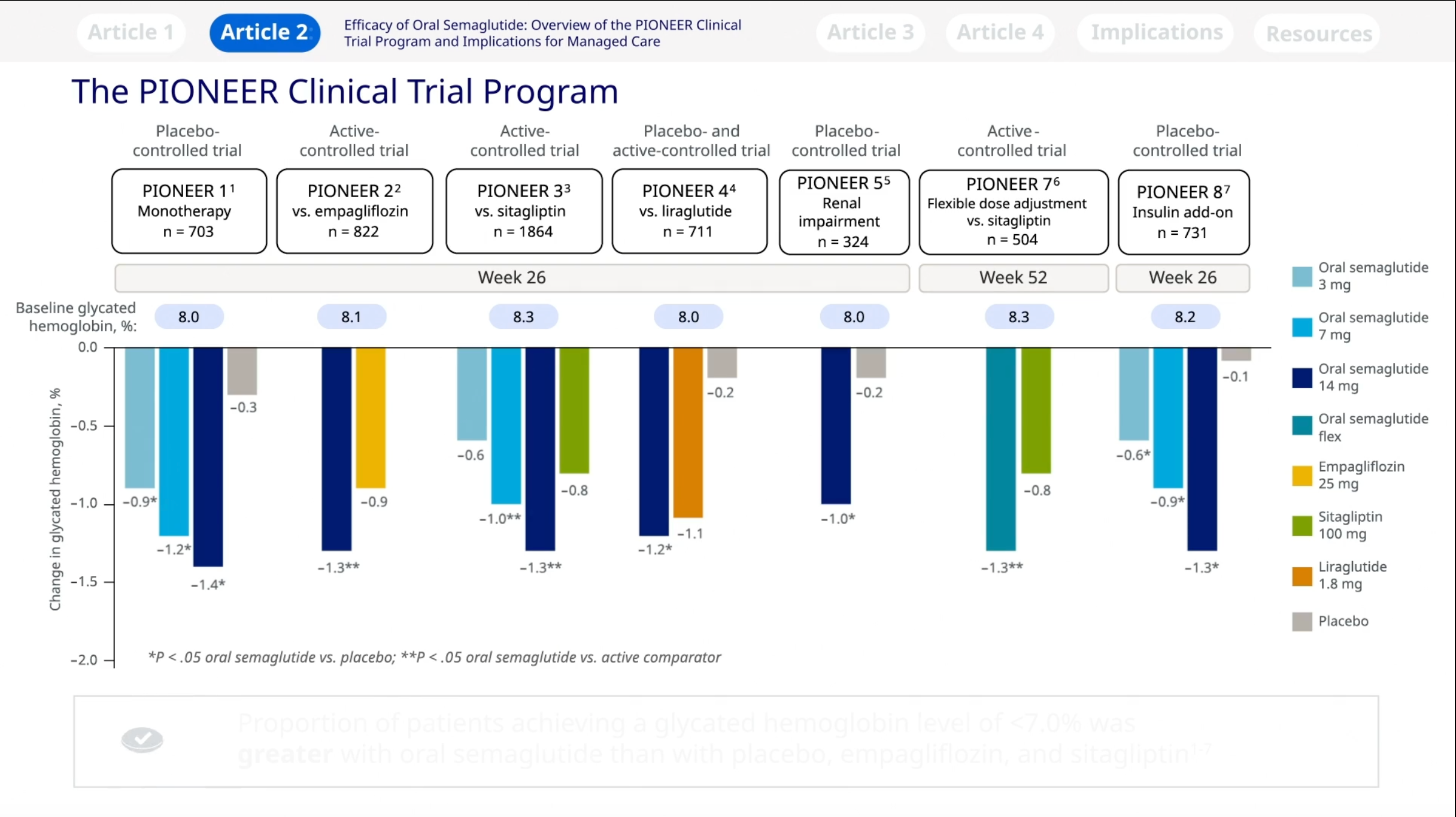
This article reviews data from the PIONEER phase 3a clinical trial program, which assessed the efficacy and sa . Patients were recruited from 149 outpatient clinics in 12 countries across .

Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, . Gastrointestinal adverse events were more frequent with semaglutide (82.Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial - The .Patients were randomly assigned, in a 3:3:3:1:1:1 ratio, to receive once-daily subcutaneous semaglutide at a dose of 0.The first tablet formulation of a glucagon-like peptide-1 receptor agonist(GLP-1RA), oral semaglutide, was approved in September 2019 for the treatment of adults with type 2 diabetes (T2D).
Safety and efficacy of combination therapy with semaglutide
4 mg, vs once-daily subcutaneous liraglutide, 3.
Objectives To compare the effects of oral semaglutide with placebo .




&quality=40&source=products/p274777_ha.jpg)


