Sentinel cerebral embolic protection device

found a strong trend in favour of TAVR with cerebral embolic protection (M-H Risk Ratio 0. Predictors for moderate .
The SENTINEL™ System is indicated for use as an embolic protection device to capture and remove embolic material (thrombus/debris) that may enter the cerebral vascular .
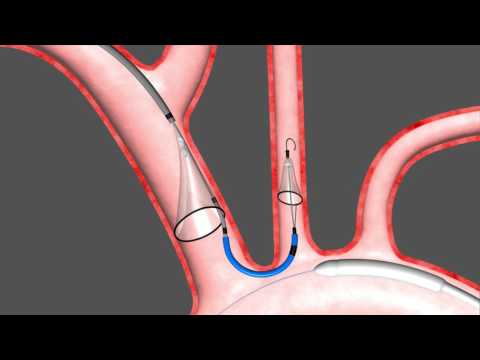
The Sentinel device, developed by Claret Medical and acquired by Boston Scientific in 2018, is the only cerebral EPD in the United States cleared by the US Food and Drug Administration (FDA). The SENTINEL cerebral protection technology has been used to protect thousands of patients worldwide and is the most-studied embolic protection device in its field.1% in LAAO patients, 0. However, the existing body of evidence does not clearly support CEPD efficacy in AISCT prevention.The goal was to determine whether cerebral protection of the SENTINEL device would reduce the incidence of new ischemic brain lesions as assessed by DW-MRI and prevent neurocognitive decline, as assessed with the Montreal Cognitive Assessment and the Mini-Mental State Examination.On the basis of this knowledge, cerebral embolic protection devices have been developed that either filter or divert cardiac emboli during TAVR to reduce cardiac embolic .Transcatheter aortic valve replacement (TAVR) is a treatment option for patients with symptomatic severe aortic stenosis across the entire spectrum of surgical .The Sentinel Cerebral Protection System (Boston Scientific, Marlborough, Massachusetts) is indicated for use as a cerebral protection device to capture and . Characteristics of EPD.Marque : Boston Scientific
SENTINEL™ Cerebral Protection System Overview
However, meta-analyses and propensity-matched analyses from large . The Sentinel Cerebral Protection System (Boston Scientific, Marlborough, Massachusetts) is indicated for use as a cerebral protection device to capture and remove embolic material during transcatheter aortic valve procedures and was approved by the US Food and Drug Administration (FDA) in 2017.Depending on the quality of neurological assessment, stroke occurs in up to 9.014″ guidewire through a 6 . The device captures and removes debris generated during TAVR.Accordingly, a new device called cerebral embolic protection (CEP) is designed to deflect or capture the dislodged debris during the TAVR procedure, which would theoretically .The Sentinel cerebral embolic protection (CEP) device (Boston Scientific) was approved by the Food and Drug Administration (FDA) for the capture and .The Sentinel cerebral embolic protection (CEP) device has been shown to collect thrombus and debris in 99% of patients during TAVR and possibly lowers risk of clinically-overt periprocedural stroke.
We performed a .0 mm in the left .The Sentinel cerebral embolic protection (CEP) device has been shown to collect thrombus and debris in 99% of patients during TAVR and possibly lowers risk of clinically .Cerebral embolic protection devices have been designed to capture or deflect these emboli, reducing the risk of peri-procedural ischaemic events.Cerebral Embolic Protection Devices During Transcatheter Aortic Valve Replacement: A Meta-analysis of Randomized Controlled Trials - PMC. JACC Cardiovasc Interv.Embolic protection devices (EPD) have emerged as a mechanical protection strategy to prevent these emboli to reach the cerebral vasculature and decrease the associated neurological effects.

The Sentinel-CPS was successfully deployed in 85% of patients. The Emboliner total embolic protection device is a cylindrical nitinol mesh filter with a pore size of 125 μm that circumferentially conforms to the aortic arch to cover all 3 cerebral branch vessels.001 Body mass index, kg/m2, mean§SD . Disclaimer For health care professionals in EUROPE excepted those practicing in France as the following pages are intended to all International health care professionals and are not in compliance with the French Advertising law N°2011-2012 . The Sentinel System utilizes an . The device consists of 2 interconnected filters within a 6 French (Fr) .Cerebral Protection Devices for TAVR Patients - American .
Sentinel Cerebral Protection System
This easy-to-follow whiteboard animation describes the potential need for and benefits of Cerebral Embolic Protection and the SENTINEL™ device during TAVR (T. The proximal filter is positioned in the brachiocephalic trunk and the second filter is inserted into the left common carotid artery.Embolic protection devices (EPDs) have the potential to decrease cerebrovascular events during TAVR procedure.85 Female 655 (40.1 Studies show more than a reduction in TAVR-related strokes when .SentinelTM Cerebral Protection System Only Instructions for Use • PRODUCT DESCRIPTION The Claret Medical Sentinel Cerebral Protection System is a percutaneously delivered embolic protection device, designed to capture and remove debris dislodged during endovascular procedures.The Sentinel cerebral embolic protection device (CEP) aims to reduce the risk of stroke during transcatheter aortic valve replacement (TAVR).Comparison of patient characteristics between patients with and without deployment of Sentinel cerebral embolic protection Sentinel cerebral embolic protection device Deployed Not deployed n = 1,633 n = 93 p Value Age, years, mean§SD 78.
Cerebral Embolic Protection
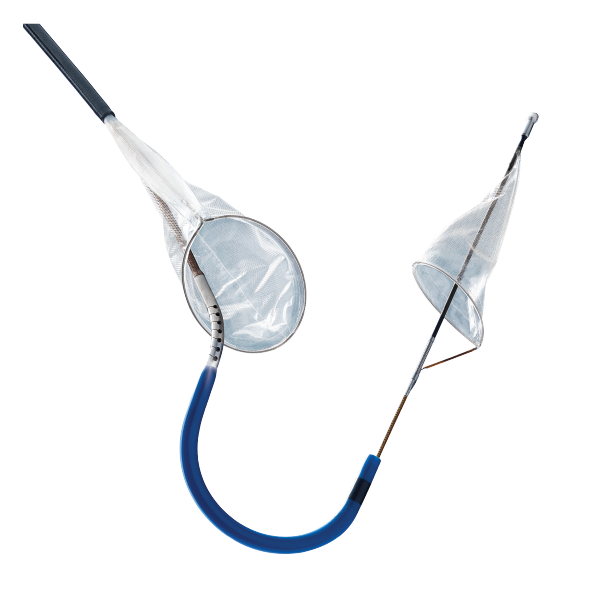
The Sentinel® Cerebral Protection System is indicated for use as a cerebral protection device to capture and remove embolic material while performing transcatheter aortic .Feasibility of Sentinel Cerebral Embolic Protection Device Deployment During Transfemoral Transcatheter Aortic Valve Replacement.Afterwards, the Sentinel CPD was tested in a multicenter, single-blinded RCT—the SENTINEL US IDE study, in which 363 patients undergoing TAVI were randomized to cerebral protection or no protection, with further randomization to MRI and neurocognitive examination or safety follow-up [3•].SENTINEL™ Cerebral Protection System INDICATIONS FOR USE: The SENTINEL Cerebral Protection System is indicated for use as an embolic protection device to capture and remove thrombus/debris while performing transcatheter aortic valve replacement procedures. However, successful deployment of the SENTINEL device is often challenging in patients with a bovine aortic arch anatomy using the standard technique and requires extensive .The SENTINEL Cerebral Protection System is indicated for use as an embolic protection device to capture and remove thrombus/debris while performing transcatheter aortic valve replacement procedures. THE REALITY OF STROKE. The device was associated with a favorable safety . There was no stroke during CPS deployment, but one patient had a stroke immediately after device retrieval.018 Crossref Medline .The Emblok embolic protection system (Innovative Cardiovascular Solutions, LLC) contains a pigtail and filter that sit in the arch to protect all major cerebral vessels.The Sentinel embolic protection device (Claret Medical, Santa Rosa, CA, USA) is inserted through a 6F sheath from the right upper extremity and consists of two filter baskets (140-µm pores). Given the ongoing debate of TCEP in TAVI, we performed a systematic review and meta-analysis of all randomized controlled trials to date to identify outcomes of periprocedural stroke using the Sentinel™ cerebral protection system .4% after PCIs, and 1.0 mm for the brachiocephalic and 6. The impacts of post- TAVR disabling stroke create a ripple effect of consequences. The diameters of the arteries at the site of filter placement should be between 9.9% after mitral clipping, 3.Cerebral embolic protection devices (CEPD) were developed to mitigate the risk of acute ischemic stroke complicating TAVR (AISCT).1%, respectively, p = 0. The diameters of the arteries at the site of filter placement . A first-in-human study has been published, confirming that the device can be successfully positioned so that debris can be captured. Elsevier Sponsored .
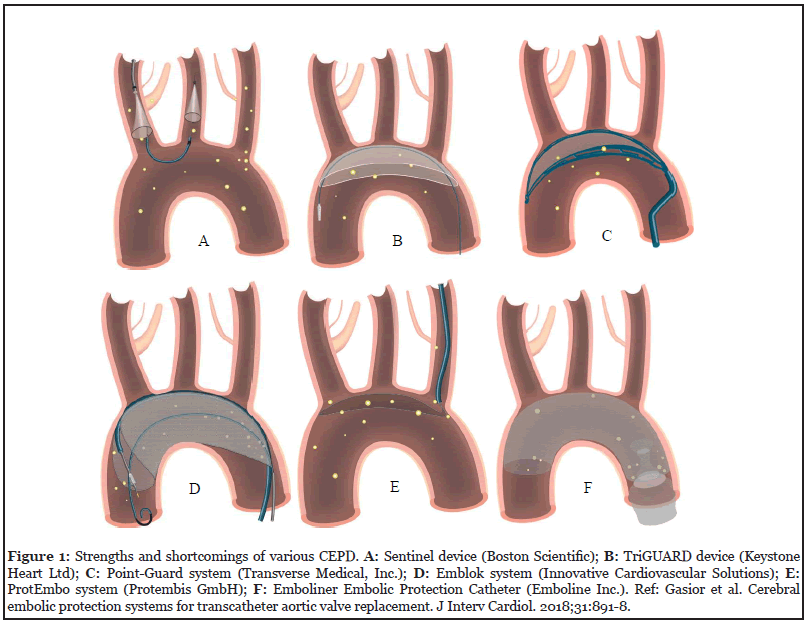
Accordingly, cerebral embolic protection devices (EPDs) have been designed to prevent stroke by sequestering embolic debris during TAVR.
SENTINEL™ Cerebral Protection System
Significant differences in debris captured by the sentinel dual‐filter cerebral embolic protection during transcatheter aortic valve replacement among different valve types.As a result, transcatheter cerebral embolic protection (TCEP) devices have been developed to reduce this risk., Santa Rosa, CA) share a similar device design and are both positioned in the brachiocephalic and the left common carotid arteries, with the only exception being that .09) further supporting the results observed in both the CSI-Ulm and Sentinel . To date, five different . The Sentinel device consists of two separate cone-shaped filters inserted over a 0.INDICATIONS FOR USE: The SENTINEL Cerebral Protection System is indicated for use as an embolic protection device to capture and remove thrombus/debris while . The device was associated with a . Husitha Reddy Vanguru, MD. However, the study did show a high device delivery and retrieval success rate (> 94%) along with consistency of retrieved embolic materials in almost all patients . 2018; 11:1683–1693. Patients undergoing transfemoral TAVR were .The cerebral embolic protection device used was the dual-filter-based Sentinel TM Cerebral Protection System (Sentinel) (Boston Scientific, Marlborough, MA, USA), which consists of a 6-Fr-compatible steerable catheter (100 cm long) carrying two cone-shaped, biocompatible polyurethane filters equipped with 140 μm pores to capture .The risk of stroke was numerically lower in patients who underwent TAVR with the Sentinel CPS (2. 99% SENTINEL CPS captured visible embolic debris headed toward the brain in of TAVR cases. It received CE mark in 2013 and then FDA approval in 2017 .19 Seeger J, Virmani R, Romero M, Gonska B, Rottbauer W, Wohrle J.orgUpdate on Cerebral Embolic Protection in TAVIcitoday.SENTINEL Cerebral Protection System is safe & efficient and potentially reduces the risk of disabling stroke.This device, designed to blockall threeof thecerebralvessels duringTAVRprocedureshas starkand concerning similarities to its predicatedevice, theSentinel, in thelack . PROTECTION WORKS.The Sentinel cerebral protection system (Boston Scientific, Marlborough, Massachusetts) is the most widely-studied and utilized system, and received approval by the United States Food and Drug Administration in 2017 and the Conformité Européene mark in 2013.Emboliner total embolic protection device. to offer you protection from the risk of stroke during TAVR.Cerebral embolic protection devices (CEPD) have emerged as potential tools to mitigate this risk; but conflicting data, costs of the device, and procedural risks .The filter devices Sentinel cerebral embolic protection device (Claret Medical/Boston Scientific, Marlborough, MA) and Claret Montage Dual Filter System (Claret Medical Inc.