Sgs belgium notified body

Ensure the conformity of your electrical products with a world leader.What is a notified body? Which medical devices require a notified body? What kind of documents are required? How do I find a Notified Body? Germany.
CE-Marking
To search for notified bodies for medical devices, follow the instructions below: 1. SGS Belgium NV Noorderlaan 87 BE-2030 .The Single Market Compliance Space (SMCS) is the system, which combines the New Approach Notified and Designated Organisations (NANDO) and the Noise Emissions by .SGS Belgium has been designated as a notified body under the EU Medical Device Regulation.Verify the notified body confirmation letters issued according to European Regulation 2023/607 extending MDD certificate validity for SGS MDD clients. Here you can browse the details of Notified Body Confirmation Letters, issued by SGS, . Bouwinspectiebureau BouwQ en SGS hebben een samenwerkingsovereenkomst .Some of them require a Notified Body to be involved.600 medewerkers werken samen in een netwerk van 2. Based on the information we received from you, SGS Belgium NV is a Notified Body for your range of products and certification will be undertaken as Notified Body 1639.On this page you will find information about the basic requirements and legislation for medical devices in the EU, and the services that SGS can provide with its Notified . The rules and requirements of the directives are .CE-marking services from SGS – meet the compulsory product safety directives you need to trade in the EU with effective conformity assessment services from a notified body.
Characteristics of the CEBEC-mark The SGS Belgium - Division SGS CEBEC certification system is an example of a type testing certification: typical samples, presenting all design elements, are investigated; production .
SGS : We are a Notified Body for the EU Medical Device Regulation
SGS Belgium - Division SGS CEBEC is a Notified Body in the framework of the both the Low Voltage Directive and the EMC Directive.usMDR and IVDR Updates – Q3 2020 | SGSsgs. Click the link entitled “Search by legislation”.Auf dieser Seite finden Sie Informationen über die grundlegenden Anforderungen und die Gesetzgebung für Medizinprodukte in der EU und die Dienstleistungen, die SGS mit Ihren Benannten Stellen (Notified Body) NB 0598 /SGS Fimko und NB 1639 / SGS Belgium anbieten kann.List of Notified bodies (certified labs) last updated version: January 2021.BouwQ en SGS starten samenwerking op het gebied van bouwinspecties.We are pleased to confirm that our Belgian Notified Body has been designated by the European Commission and Belgian Competent Authority (FAMHP) under Medical Device Regulation (EU) .After the successful migration of our client’s MDD certificates to SGS Belgium (Notified Body 1639) in March 2020, our primary focus has been the MDR designation for SGS Belgium.
List of NOTIFIED BODIES
With more than 93,000 employees, we operate a network of over 2,600 offices and laboratories around . Click the link entitled “Notified bodies”.SGS Belgium NV is a Notified Body with a notification scope described in the official European Nando database. We are the world's leading testing, inspection and certification company.Head of Notified Body 1639, Certification Knowledge Solutions t: +32 3 545 48 60.

Notified bodies (NANDO) Notification is an act whereby a Member State informs the Commission and the other Member States that a body, which fulfils the relevant .
SGS (Belgium) Notified Body Reviews
KONHEF vzwGijzelaarsstraat 7-9 . We are the world's .comRecommandé pour vous en fonction de ce qui est populaire • AvisSGS Belgium NV has been the latest NB notified under the Regulation (EU) 2017/745 on medical devices – MDR.List of Notified bodies accredited for Medical Device CE .

With a presence in Belgium’s three major ports in Antwerp, Zeebrugge and Ghent, we have become a trusted provider of inspection, verification, certification and testing services in . This means you are entitled to . These are user-submitted reviews of medical device companies describing their experience working with this notified body.SGS is erkend als de global benchmark voor kwaliteit en integriteit.SGS Belgium NV is a Notified Body for Class III and IIb devices, and certification will be undertaken as Notified Body 1639. The CEBEC certification system is a example of a type testing certification: typical samples, presenting all design elements, are investigated; production is surveyed by regular inspections;
MDR and IVDR Updates
Explore SGS's Comprehensive Medical Device Services at Booth 702: Regulatory Expertise: Learn about our role as a Notified Body, offering CE marking through our .600 kantoren en laboratoria om een .ce-certification.

comThe database of notified bodies (NANDO) | FPS Economyeconomie.SGS is a Medical Device Notified Body for your range of products and certification will be undertaken as Notified Body 1639 for SGS Belgium NV. Under the column “Legislation name” find the link entitled “Regulation (EU) 2017/745 on medical devices”.
Appointment of a new Belgian Notified Body EU MDR
As a world-leading provider of testing, inspection, verification and certification, we offer you unrivaled experience in electrical .

CE Type examination certificates issued as EU Notified Body in the frame of EU Regulation (CPR) EU 305/2011, or EU Directive (EMC) 2014/30/EU. Go to the above-mentioned link. This means that you are entitled to use CE 1639 on devices covered by your EU technical documentation assessment certificate, on completion of a successful assessment.SGS Belgium NV - Division SGS CEBECBld International 55/D1070 BRUXELLESCountry : Belgium Notified Body number : 0649. Class III, implantable class IIb 1 and class IIb .
SGS in Belgium

SGS : We are a Notified Body for the EU Medical Device Regulation. Although we have reached the final stages, the COVID-19 pandemic has had a serious impact on the work of member state Competent Authorities .SGS Belgium NV, a Notified Body (NB 1639) designated against European Regulation 2017/745 (MDR), issues a Notified Body Confirmation Letter to an exisiting SGS MDD client when a formal transition application is received and a written agreement is signed. Please note that devices covered by Annex IX Section 5 .
25 MDR Notified Bodies & EUDAMED Implementing Act

European Regulation 2023/607, published in March 2023, extends MDD validity by up to five years.
Medical device questionnaire
SGS Belgium NV, a Notified Body (NB 1639) designated against European Regulation 2017/745 (MDR), issues a Notified Body Confirmation Letter to an exisiting SGS MDD client when a formal transition application is received and a written agreement .Reviews of SGS, a Notified Body in Belgium.
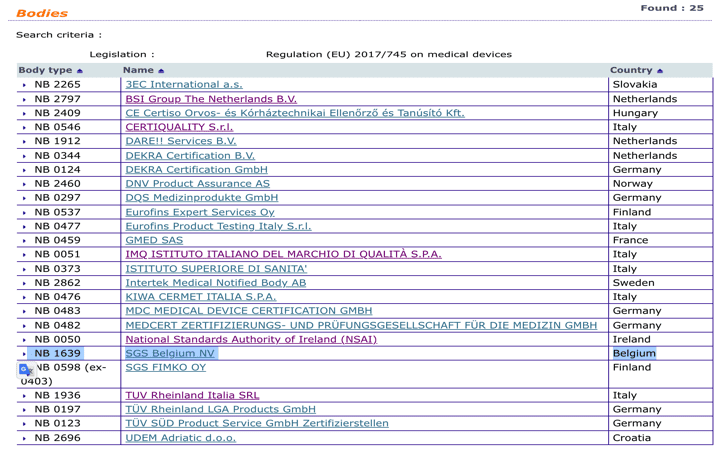
NB number Notified bodies Country; 0462 .beNotified Bodies in the European Union: A Complete Guide - . We are happy to inform you that the .For that reason, a few months ago, we decided to transfer our notified body from SGS UK (CE0120) to SGS Belgium (CE1639).SGS Belgium NV is aangewezen als Notified Body voor de nieuwe EU-Verordening betreffende medische hulpmiddelen (MDR) December 03, 2021 We zijn blij te kunnen bevestigen dat onze Belgische Notified Body is aangewezen door de Europese Commissie en de voor aangemelde instanties verantwoordelijke Belgische autoriteit .
Notified Body in Belgium
SGS Annual Report Job Opportunities Upcoming Webinars Sustainability Solutions.With the transition in Europe from the Medical Device Directive to the new Medical Device Regulation, we have gained MDR Notified Body status. We are pleased to confirm that our Belgian .