Shi asymmetric epoxidation
An asymmetric epoxidation using a fructose-derived chiral ketone. It can be used on a large scale, . To read the full-text of this research, you can .
Shi Epoxidation: A Great Shortcut to Complex Compounds
1980 , 102 , 5974).
Myers Shi Asymmetric Epoxidation Reaction Chem 115
Reviews: Wong, O.史氏环氧化反应(Shi Epoxidation) 反应机理下载链接: chem. 2 Shi has also developed a very efficient method for asymmetric epoxidation, using a ketone-derived organocatalyst based on d-fructose ().The ‘Sharpless asymmetric oxidation’ is achieved with the use of a chiral catalyst composed of (+) or (-) diethyltartrate and an organotitanium compound (J.Several generations of chiral ketones have been developed to realize the asymmetric epoxidation of each type of unfunctionalized alkenes, including trans‐, . Bristol-Myers Squibb. Chemical Reviews 2014, 114 (16) , .The asymmetric epoxidation of olefins using chiral dioxirane generated in situ from Oxone (potassium peroxomonosulfate KHSO 5:KHSO 4:K 2 SO 4 =2:1:1) and .338-339) Authors: Jack Li. Andrea Wong, Bin Wang, Mei-Xin Zhao and Yian Shi.The reaction is stereospecific and decomposition of H 2 O 2 has not been observed. The topic is also known as: Shi asymmetric epoxidation.Review the Shi asymmetric epoxidation where alkenes are treated with oxone in the presence of a fructose-derived ketone catalyst known as Shi’s catalyst. Organocatalytic Oxidation.There had been many previous attempts to create an efficient non-metal catalyst for asymmetric epoxidations. The Shi epoxidation involves treating alkenes with oxone (potassium . 2004, 37, 488–496. This reaction is commonly referred to as the Shi epoxidation and the Shi asymmetric epoxidation . This reaction is thus known as the Shi epoxidation in general and the Shi asymmetric epoxidation on occasion.The asymmetric epoxidation of olefins using chiral dioxirane generated in situ from Oxone (potassium peroxomonosulfate KHSO 5:KHSO 4:K 2 SO 4 =2:1:1) and the ketone derivative of fructose-acetal. Popular works include An Efficient Catalytic Asymmetric Epoxidation Method, An Efficient Asymmetric Epoxidation Method for trans-Olefins Mediated by a .
有机人名反应——史氏环氧化反应(Shi Epoxidation)
This article describes a highly effective catalytic asymmetric epoxidation method for olefins using potassium peroxomonosulfate (Oxone, Dupont) as oxidant and a .Since the introduction of the Shi catalyst, the organocatalytic epoxidation of olefins has become an area of continued research interest. Uses of the Reaction • The Sharpless Asymmetric Epoxidation converts alkenes into chirally active epoxides • . In book: Name Reactions (pp., 2008, 73, 9539-9543.LiteratureJacobsen-Katsuki EpoxidationSharpless EpoxidationBaeyer-Villiger OxidationPrilezhaev ReactionSynthesis of Epoxides
Shi Asymmetric Epoxidation
For example, hexe-2-en-1-ol undergoes epoxidation to give chiral epoxy alcohols with 94% ee and 85% yield in presence of 5-10 mol% of Ti (O i Pr) 4 , L- (+)-DET and t -BuOOH (Scheme 5.


Shi asymmetric epoxidation.

Skip to main content. Oxidation of the catalyst with Oxone to generate the active epoxidation reagent. Organic Letters 2018, 20 .Jacobsen Epoxidation Jacobsen-Katsuki Epoxidation. Andrea Wong, Mei-Xin Zhao and Yian Shi.
An Efficient Asymmetric Epoxidation Method for
An asymmetric epoxidation using a fructose-derived chiral . The use of Ti(salan) for the epoxidation of alkenes has been demonstrated in the presence of aqueous H 2 O 2. Since this discovery, the use of Shi’s catalyst has become known as the Shi asymmetric . The Journal of Organic Chemistry 2008, 73 (24) , 9539-9543. In 1996, Yian Shi of Colorado State University developed a fructose-derived ketone catalyst that demonstrated excellent enantioselectivity. Organocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic Applications.Shi epoxidation provides an extremely powerful tool to access optically active epoxides, which undoubtedly belongs to one of the earliest and most successful .The Sharpless asymmetric epoxidation of allylic alcohol provides a powerful tool for the synthesis of optically active epoxy alcohol.In the early stages of their synthesis of the sesquiterpenoid sagittacin E (27), Abe, Ito, and coworkers made use of a Shi epoxidation for asymmetric desymmetrization of the .
Shi Epoxidation
Since this discovery, the use of Shi’s catalyst has become known as the Shi asymmetric epoxidation.
Andrea Wong and Yian Shi. The Jacobsen Epoxidation allows the enantioselective formation of epoxides from various cis-substituted olefins by using a chiral Mn-salen catalyst and a stoichiometric oxidant such as bleach.• The Sharpless Asymmetric Epoxidation converts alkenes into chirally active epoxides • Innumerable syntheses published that use the SAE • Chiral epoxides easily converted . 2008, 108, 3958–3987.
Shi Epoxidation
First Online: 01 January 2014.Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by .3 Titanium-Catalyzed Epoxidation with Hydrogen Peroxides. The resulting sulfate intermediate is a good leaving group that facilitates the ring closure to . Asymmetric Epoxidation of 1,1-Disubstituted Terminal Olefins by Chiral Dioxirane via a Planar-like Transition State.Shi epoxidation is a research topic. 2 Shi has also developed a very efficient method for asymmetric epoxidation, using a ketone-derived organocatalyst based on d-fructose . first reported the asymmetric epoxidation of olefins using chiral dioxirane generated in situ from Oxone (potassium peroxomonosulfate, KHSO 5 : KHSO 4: K 2 SO 4 = 2 : 1 : 1) in 1996.1021/jo801576k.

The Shi epoxidation involves treating alkenes with oxone (potassium peroxymonosulfate) in the presence . Tetrahedron Letters 2006 , 47 (34) , 6079-6082.A new catalytic system for the asymmetric epoxidation of allylic alcohols has been developed featuring high enantioselectivity for Z olefins, catalyst loading of less than 1 mol%, reaction temperatures of 0°C to room temperature over a shorter time, use of aqueous tert-butyl hydroperoxide (TBHP) instead of anhydrous TBHP as an achiral oxidant, and .

Auteur : Xiangqing Feng, Haifeng Du
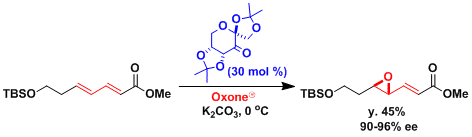
Asymmetric Epoxidation Using Shi Catalyst
Enantioselective .1007/978-3-662-04835-1_262. The most striking feature of this system is aliphatic alkenes that are one of the most challenging . The reaction proceeds under basic conditions and within a mild temperature range (0℃~rt). Chapter; First . Asymmetric Epoxidation Catalyzed by α,α-Dimethylmorpholinone Ketone. Published in Catalysis from A to Z 18 April 2020.Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless 1 and Jacobsen-Katsuki.Shi asymmetric epoxidation. Reaction Mechanism. Methyl Group Effect on .5 R 2 R 1 O O O O O O H3C CH3 O H3C CH3. Over the lifetime, 55 publications have been published within this topic receiving 2100 citations. Find a journal Publish with us Track your research Search. An asymmetric epoxidation using a fructose-derived .The Shi asymmetric epoxidation allows the synthesis of epoxides from alkenes using a fructose-derived organocatalyst with Oxone. Several generations of chiral ketones have been developed to realize the asymmetric epoxidation of each type of unfunctionalized alkenes, including .Shi Asymmetric Epoxidation Reaction. Since this discovery, the use of Shi’s catalyst has become known as the Shi asymmetric epoxidation. It is an organocatalyst with Oxone typically used as the primary oxidant.Shi Epoxidation • Reaction involving a trans alkene • Oxone is another main component • Fructose derived catalyst used • High enatiomeric excess yields R 1 R 2 Oxone H 2 O,CH 3 CN pH 10. Tetrabromobisimidazole (±)- 4a was prepared by the procedure in our previous .