So4 charge formula
Here, sulphur is the central atom, and it is surrounded by four oxygen atoms that are located at equal distances in the plane.Formula and structure: The sulfate ion formula is SO4 2-and the molar mass is 96. En ce qui concerne sa masse, seule celle du noyau est considérée.Regarder la vidéo11:30We draw the dot structure in the exact same manner, and then calculate the formal charges for the atoms in the molecule.Il s'agit d'un sel d'ammonium NH 4 + et d'acide sulfurique H 2 SO 4 qui possède de très nombreuses applications. Add or subtract electrons if the structure is an anion or cation, respectively. 2 to see how to perform this calculation.comRecommandé pour vous en fonction de ce qui est populaire • Avis
And Why?
Because these ions contain more than one atom, they are called polyatomic ions. By formula: O4S-2+12 H 2 O = H 24 O 16 S -2. Remember that formal charge is calculated by taking the # of . Create: 2004-09-16. Molecular Weight.L'équation balancée. Add an electron for every negative (-) charge, and . At the heart of comprehending the characteristics and reactivity of SO4 2- lies the exploration of its Lewis structure. Ce composé se présente sous la forme de cristaux vert pâle ; c'est un sous-produit du fer de décapage. Modify: 2024-04-16.5* Bonded Electrons.Molecular Formula. Beware that this must be the lowest whole # ratio of cation to anion. Example \(\PageIndex{1}\) Write the chemical formula for an .
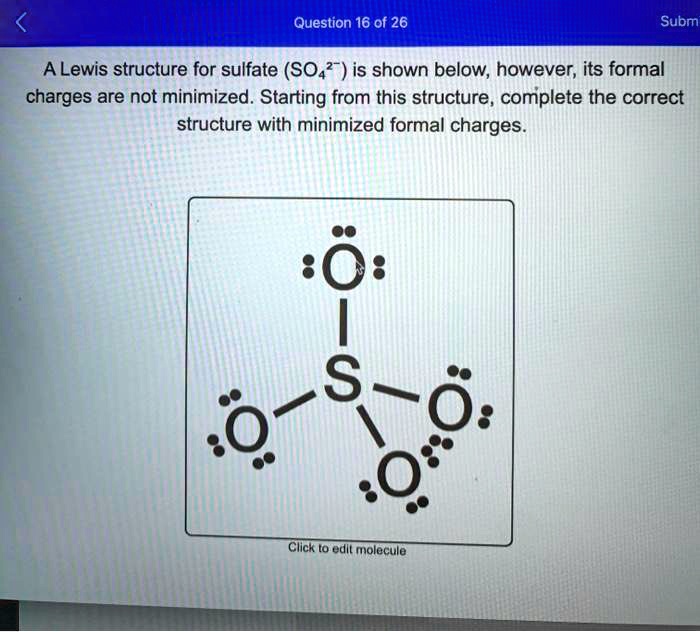
Lewis Structure of SO4(2-) (Sulfate) CORRECT
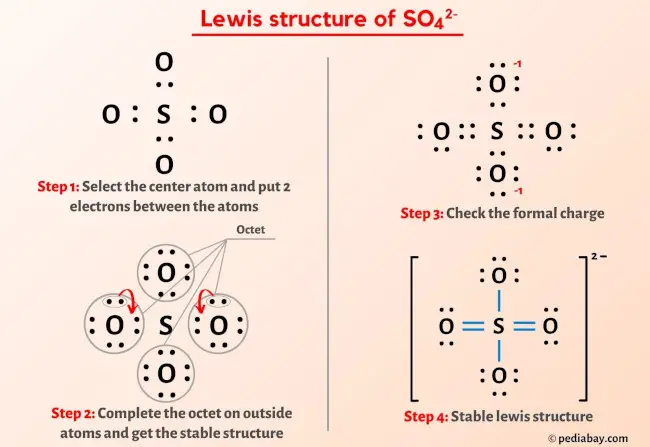
Step #5: Check the formal charge. But, in order to get the most stable lewis structure, we have to check the formal charge on SO4 2-ion.A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ).To do this, we need to use the below formula: Formal Charge= Valence Electrons- Lone pair electrons- 0. Austin State University) with contributing authors.What is sulfate in chemistry?Sulfate is considered a polyatomic anion. There are two ways to determine the charge on the Sulfate ion (SO4 2-). For the sulfur . Be sure to have the correct number of electrons.Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom.The SO4 2- (Sulfate Ion), comprised of one sulfur atom and four oxygen atoms, presents a captivating example of a chemical species with intriguing properties.Introductory Chemistry. What is a Sulfate? Sulfate is a compound. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
SO42- Formal charge, How to calculate it with images?
Through a combination of explanations, visual aids, and frequently asked .
Il est notamment couramment utilisé comme engrais destiné à l'acidification . For each oxygen atom, Formal Charge= 6- 6- 0. the formal charges are closest to 0 (and also the second structure does not give a complete octet on N) Contributors Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Il est utilisé comme .Regarder la vidéo1:599. Here, sulphur is . O 4 S-2; Synonyms. Magnesium sulfate is commonly known as Epsom salts. For example, NO 3− is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge.L'anion est un ion qui a gagné des électrons et dont la charge est maintenant négative. Celui-ci est capable de se combiner avec de l'hydrogène afin de former le sulfure d'hydrogène, un gaz toxique possédant une odeur facilement reconnaissable, celle de l’œuf pourri. Therefore, we predict that the ionic compound formed will be KBr , potassium bromide.In this video we'll write the correct formula for Sulfate ion (SO4 2-). IUPAC Standard InChI: InChI=1S/H2O4S/c1-5 (2,3)4/h (H2,1,2,3,4)/p-2.Ainsi, Total des électrons de valence dans SO4 2- ion = électrons de valence donnés par 1 atome de soufre + électrons de valence donnés par 4 atomes . A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg2+Cl− Mg 2 + Cl −. So it fulfills the octet rule.multiply charge of each ion by absolute value of the charge of the counter ion.

Sulfate is an anion beca. backgroundColor = white getValue .How to Calculate the Formal Charges for SO3 2- (Sulfite ion)youtube. Make sure resulting ratio is the lowest whole number ratio, if not, divide by common denominator so all values are integers.Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. Sulfate ions consist of one sulfur (S) atom . You can see from the above image that the central atom (i.Structural Formula. The reason is FORMAL CHARGE and the fact tha. ∴ The formal charge on each single-bonded oxygen (O) atom in [SO4]2- is -1.e sulfur), is having 8 electrons.Formal charge on the single bonded Oxygen atom = 6 – 6 – 2/2 = 6 – 6 – 1 = 6 – 7 = -1.What is the formal charge of S in SO4? The formal charge of sulfur in a sulfate compound is zero. Combining one ion of each does not completely balance the positive and negative charges. The sulphate ion is composed of sulphur and oxygen atoms.
Iron typically exhibits a charge of either 2+ or 3+ (see ), and the two corresponding compound formulas are FeCl 2 and FeCl 3.
State of charge
To determine the . étape: Écrire une équation non équilibrée ('équation squelette') qui contient tous les réactifs et produits de la réaction chimique. Pour obtenir meilleurs . A polyatomic ion is a group of two or more atoms that behave as a single unit. For example, consider binary ionic compounds of iron and chlorine. Computed by PubChem 2.

Pour finir, la taille d'un atome est très petite. 14808-79-8 ; Sulfate ; Sulphate ; Sulfate ion ; Sulfate(2-) View More. Ce sel était autrefois dénommé sel sec ou sel desséché de Glauber, car il provenait . Write the correct .The sulphate ion is mainly composed of sulphur and oxygen atoms.Les charges électriques étant les même, il y a autant d'électrons qui gravitent autour du noyau que de particules le constituant.Propriétés physiques et chimiques. - WebMDwebmd.8K views 1 year ago. For that, you need to remember the formula of formal charge; So far, we have discussed elements and compounds that are electrically .What is the formal charge of S in SO4?The formal charge of sulfur in a sulfate compound is zero. Determining Ion Charges from Formula. To balance the positive and negative charges, we look to the least common multiple—6: two iron 3+ ions will give 6+, while three 2− oxide ions will give 6−, thereby balancing the overall positive and negative charges. Chemical Formula.The charge of the metal ion is determined from the formula of the compound and the charge of the anion.comQuora - A place to share knowledge and better understand .Le sulfate d'ammonium est un corps composé chimique ionique de formule (NH 4) 2 SO 4.
Sulfate
Learning Objectives. Trick: Set # of Anions = Charge of Cation and. On dit que la masse du noyau est concentrée dans son noyau.The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that.
SO4 2- Structure de Lewis en 5 étapes (avec images)
To write the formula for Sulfate ion we must first recognize that it is a polyatomic .Formula: O 4 S -2. Valence electrons of oxygen = It is present in Group VI A = 6 valence electrons.Sulphate Formula: SO₄²⁻.In this video we'll write the correct formula for Lead (IV) sulfate, Pb(SO4)2.
How to Write the Chemical Formula for Sulfate ion
The oxygen atom will adopt a 2− charge as an ion.Critiques : 13 The first, and probably quickest is to just memorize that the Sulfate ion has a .89℃ Melting Point. Il existe dans la nature, sous le nom minéralogique de mascagnite.orgSO42- Lewis Structure (Sulfate ion)chemistryscl.State of charge ( SoC) quantifies the remaining capacity available in a battery at a given time and in relation to a given state of ageing. Generate a proper name for an ionic compound. With an empirical formula of SO 42- , this molecule carries a net charge of -2 as an anion.dans cette vidéo on peut s'intéresser à la charge format des à l'état d'oxydation ou nombre d'oxydation d'un atome mais ces deux notions c'est tout simplement deux façons . set # of Cations = Charge of Anion.
Lewis Structures
This is due to its six different resonance structures. Strategy: Identify charge of each ion. Two of the oxygens are single-bonded and two are double-bonded. Bonding electrons around Oxygen = 1 double bond = 4 electrons.HSO4−(aq) +H2O(l) ↽−−⇀ H2SO4(aq) + OH−(aq) HSO 4 − ( aq) + H 2 O ( l) ↽ − − ⇀ H 2 SO 4 ( aq) + OH − ( aq) with Kb = 1 ×10−15 K b = 1 × 10 − 15. SO₄²⁻ Molar Mass.47℃ Sulphate Structure :[SO 4-2] Let us understand the chemical bonding and molecular structure of sulphate. Lors d'une électrolyse, les anions se dirigent naturellement vers l'anode, en raison de . Now consider the ionic compound formed by magnesium and chlorine. Thus, the formula for this ionic compound is Fe 2 O 3.Write the chemical formula for a simple ionic compound. The simplest name, “iron chloride,” will, in . Molecular Model. Gregory Hodgkins (YouTube) Step 1.Lorsqu'il est à l'état anhydre, il prend l'apparence d'un solide cristallin blanc de formule chimique Na 2 SO 4 dont la forme naturelle est la thénardite des minéralogistes.comHow does SO_4 have a charge of 2-? + Example - Socratic.Le sulfate ferreux a pour formule FeSO 4.
Charge formelle et nombre d'oxydation (vidéo)
How to Draw Lewis Structures in 5 Easy Steps.orgRecommandé pour vous en fonction de ce qui est populaire • Avis
How to Find the Charge on the Sulfate Ion
(•10)+ = (•11) By formula: (O4S-2•10 H 2 O)+ H 2 O = (O4S-2•11 H 2 O) Bond type: Hydrogen bond .+12 = H 24 O 16 S -2._Canvas2D (JSmol) jmolApplet0 [x] getValue bgcolor = white.

[1] It is usually expressed as percentage .comRecommandé pour vous en fonction de ce qui est populaire • Avis
How to calculate the formal charge of sulfate ion?
eduSodium sulfate - Wikipediaen.The first structure is the best structure.Auteur : chemistNATE For double-bonded oxygen atoms.
Worked example: Finding the formula of an ionic compound
Le sulfate de sodium est un composé chimique courant formé d'un ion sulfate et de deux ions sodium.Auteur : Wayne Breslyn2 (PubChem release 2021. Pour cause, celle des électrons est presque nulle.To find the formula of an ionic compound, first identify the cation and write down its symbol and charge.
Organic sulfate esters, such as . Expand/collapse global location. These last oxygen atoms are the responsible for the negative charge (-2) of the anion.Bromine, as a group 17 halogen, usually forms anions with a 1- charge, namely, Br − . Molecular weight: 96.Formal Charge = [Number of valence electrons on atom] – [non-bonded electrons + number of bonds] I don't think I am using it correctly in finding the formal charges of each atom in SOX4X2− S O X 4 . IUPAC Standard InChIKey: QAOWNCQODCNURD . Count all the valence electrons for each atom. Sulfate ions play a significant role in various chemical processes.How to Find the Charge on the Sulfate Ion - YouTubeyoutube. For the bonding, 2 of the oxygen atoms form S=O bonds and the other two form S-O- bonds.Construct a proper formula for an ionic compound.Formal Charge Formula: Formula Charge Calculation of SO 4 2-: Importance of Formal charge: Formal Charge Definition: “The formal charge over an atom of a polyatomic molecule or ion is the difference between the valence electron of that atom in the elemental state and the number of electrons assigned to that atom in Lewis structure. 2 7 formula ionic compound.comCalculating SO42- Formal Charges: Calculating Formal .For (b), NH 4 has a charge of +1 and PO 4 has a charge of 3-, so by crossing charges the formula is (NH 4) 3 PO 4.To write the formula for Lead (IV) sulfate we’ll use the Periodic Table, a Comm.State of charge (SOC) in the batteries is related to the energy stored in a system, it can be calculated as follows [23]: (7) where σ is the self-discharging rate of . Sulfate is a sulfur oxoanion obtained by deprotonation of both OH .Critiques : 13
Sulfate Ion Formula & Structure
Critiques : 29 Voici des échantillons de soufre sous forme cristalline.Therefore, the proper formula for this ionic compound is MgO MgO.
Lewis Dot Structures
comLewis Structure for SO4 2- (Sulfate Ion) - UMDterpconnect.What is sulfate used for?Organisms living near deep-sea thermal vents use sulfates as electron acceptors.













