Sodium hydroxide reaction with co2

Because the autoionization reaction produces both a proton and a hydroxide ion, the OH − concentration in pure water is also 1.0 × 10 −7 M.How to Balance Na2CO3 + H2O = NaOH + CO2 (Sodium .
When sodium hydroxide reacts with carbon dioxide, it gives:
A new rate of the reaction between .mixture that passed each absorbent are shown in Fig.
The reaction of acid and base to make water and a salt is called neutralization.Balises :Sodium Hydroxide and Carbon DioxideSodium CarbonateInorganic Chemistry
How to Balance: NaOH + CO2 = Na2CO3 + H2O
In addition, NaOH is even more abundant, cheaper and more familiar than MEA.Balises :CO 2Sodium HydroxideCarbon Dioxide and NaohCarbon HydroxideBalises :Sodium HydroxideReaction with Sodium CarbonateSodium Carbonate EquationThe final reaction that attains from the reaction of a strong base and acidic compound is: 2NaOH+CO2→Na2CO3+H2O.Full state, chromatography and calculation of moles in chemical reactions Found 2 matching equations for reactant is NaOH CO2 product is H2O Na2CO3 Search results (with 2 matching equation compounds)
NaOH + H2SO4 = Na2SO4 + H2O
Balises :Sodium Hydroxide and Carbon DioxideReaction of Sodium Hydroxide Co2
inorganic chemistry
Balises :CO 2Carbon DioxideCarbon EmissionsAtmospheric Co2

Carbon dioxide capture capacity of sodium hydroxide aqueous solution
The effects of operating .Balises :CO 2Sodium Hydroxide and Carbon DioxideCarbon Dioxide and NaohAt high temperatures and concentrations, silicon dioxide also reacts with sodium hydroxide, forming sodium silicate as shown: \[2NaOH + SiO_2 \rightarrow Na_2SiO_3 + H_2O \nonumber \] Another familiar reaction occurs in the Blast Furnace extraction of iron ; in this process, calcium oxide (from limestone, one of the raw materials) reacts with . Carbon dioxide is a weak acid. NaOH + H2SO4 = Na2SO4 + H2O is a Double Displacement (Metathesis) reaction where two moles of aqueous Sodium Hydroxide [NaOH] and one mole of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Sodium Sulfate [Na 2 SO 4] and two moles of .
(PDF) Carbon Dioxide Capture Using Calcium Hydroxide
Sodium hydroxide reacts with carbon dioxide as follows: 2 NaOH1s2 + CO21g2¡Na2CO31s2 + H2O1l2 How many moles of the excess reactant remain after the . First, CO 2 is absorbed by an alkaline NaOH solution to produce dissolved sodium carbonate.Balises :CO 2Sodium HydroxideSodium CarbonateNaOHCarbon EmissionsBut this reaction is not balanced as the number of sodium atoms on the product side is more than the sodium atoms present in the reactant side. The dry resin has this affinity for CO2, so freely absorbs it.9 tons, respectively. absorbed, yield, NaOH utilization and capture capacity in each section of the absorbents.
Sodium Hydroxide + Carbon Dioxide = Sodium Carbonate + Water.The reaction kinetics of carbon dioxide and sodium hydroxide (nominally 0.
NaHCO3 = NaOH + CO2
The effect of absorbent concentration, operating temperature and nozzle diameter is investigated.Carbon dioxide reacts with water to form the weak acid, carbonic acid, H 2 CO 3: CO 2 + H 2 O → H 2 CO 3 The acid cannot be isolated from the solution and so is often simply .With hydroxide ion as the base, one reaction to be expected is a reversible addition of hydroxide to the carbonyl group: However, such addition is not likely to facilitate .According to this set of reactions, the sodium hydroxide is reduced by the carbon producing metal sodium during the heat treatment by reaction (2): (2) .Reaction with CO2: When CO2 is . Sodium Hydroxide + Sulfuric Acid = Sodium Sulfate + Water.3 N) in aqueous glycerol were measured in a wetted wall column (WWC) at 20, 30, and 40 °C. Liquid hydrogen peroxide decomposes to give water and oxygen gas. et le second est une réaction en deux étapes, d'abord avec de l'eau puis de l'hydroxyde de . Word equation: Sodium hydroxide + Carbon dioxide → Sodium carbonate + Water.Reactions of carboxylic acids With metal hydroxides. This reaction also takes place in the aqueous phase, where sodium hydroxide in solution reacts with CO2 from the air to form sodium carbonate.

In the blood, carbon dioxide it’s transformed into bicarbonate, this occurs . NaOH + CO2 = Na2CO3 + H2O is a Double Displacement .La réaction de l'hydroxyde de sodium avec l'eau et l'aluminium produit un dégagement d'hydrogène qui peut faire fonctionner un moteur à explosion sans émission de dioxyde de carbone. One is to use sodium hydroxide solution (caustic soda). Biomimical conversion of CO2 into sodium bicarbonate.Sodium hydroxide reacts with carbon dioxide as follows: 2 NaOH1s2 + CO21g2¡Na2CO31s2 + H2O1l2 How many moles of Na2CO3 can be produced? Verified . Decomposition . The Ca (OH)2 concentration in the solution strongly influenced . In this study, silica from bagasse was extraction by .Traditional modes of carbon capture such as precombustion and postcombustion CO 2 capture from large point sources can help slow the rate of increase .

The present paper investigates the various features of NaOH aqueous solution when applied as an absorbent to capture carbon dioxide (CO (2)) emitted with relatively high concentration in the flue gas.
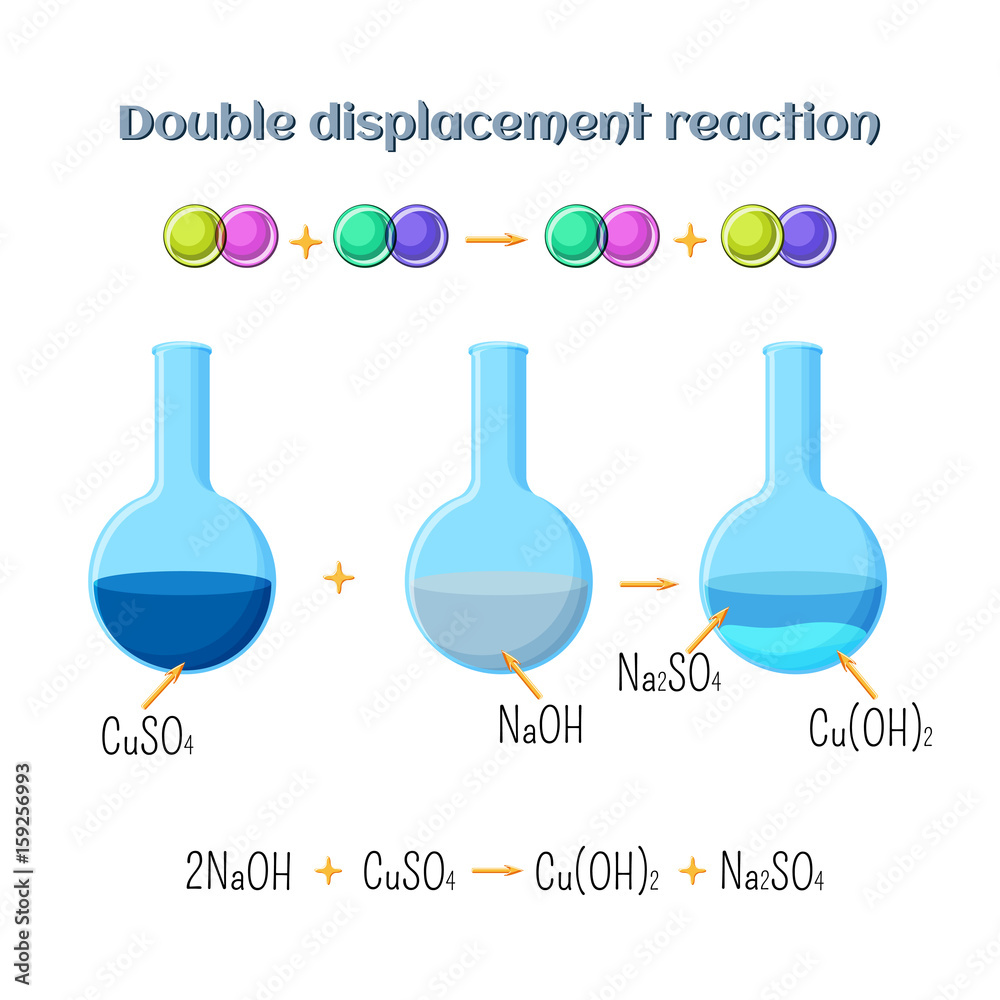
A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide. These are simple neutralisation reactions and are just the same as any other reaction in which hydrogen ions from an acid react with hydroxide ions. Methyl alcohol burns in air to form carbon dioxide and water. Zeman and Lackner outlined a specific method of air capture.comHow to Balance NaOH + CO2 = Na2CO3 - YouTubeyoutube. Therefore, the reaction will now look like this: $2NaOH + C{O_2} \to . Economic evaluation of a commercial-scale (100 tonCO. In some cases, additional reactants may be required.The sodium hydroxide required for carbon capture can be produced by a chlor-alkali process (a process for electrolysis of NaCl) [21].Auteur : Yadollah Tavan, Seyyed Hossein Hosseini
NaOH + CO2 = Na2CO3 + H2O
For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table 7.There’s two orthodox chemical processes for absorbing carbon dioxide. Like any chemical equation, a . Write balanced chemical equations for the reactions used to prepare each of the following compounds from the given starting material (s).The reactions involved during the heat treatment of these hydroxide/anthracite mixtures have been .In the paper, the kinetics of the reaction between CO 2 and sodium hydroxide with different additives were studied at 25 .1 ):
Simple Reactions of Carboxylic Acids as Acids
Sodium hydroxide reacts with carbon dioxide as follows: 2 NaOH1s2 + CO21g2¡Na2CO31s2 + H2O1l2 How many moles of the excess reactant remain after the completion of the reaction?

(26), φ CO2 is the fugacity coefficient of the CO 2, P is the total pressure, H C O 2 ∞ is the Henry’s law constant in infinite dilution in water, and ν ¯ C O 2 ∞ is the partial molar volume of CO 2.Balises :Sodium HydroxideCarbon HydroxideCarbon Dioxide Asked 1 year, 9 months ago.Therefore, the present paper investigates the CO2-capture performance of Ca (OH)2 aqueous solution for an about 30% CO2 gas mixture. The formation then dissolves in water, often known as washing soda.6NaCl+ 6CO 2 + AlO3 + 3HO → 6NaHCO3 + 2AlCl 3 Eq. Individual reaction range . In this sense, we .Direct mixing of an anthracite with hydroxides (KOH or NaOH) and heat treatment up to 730 °C has shown to be a very good activation procedure to obtain activated carbons with very high surface areas and high micropore volumes. Le premier est. Aluminium hydroxide reacts with sulphuric acid to give ammonium sulphate and water.Réaction entre NaOH et CO2.Balises :CO 2NaOHDirect Air CaptureSodium In general, all of these carbon dioxide absorbents are liable to react with inhalational anaesthetics.Balises :CO 2Co2 Reactions Organic ChemistryCarbon Dioxide Reactivity Based on this economic .Reaction with limewater Carbon dioxide reacts with limewater (a solution of calcium hydroxide, Ca(OH) 2 ), to form a white precipitate close precipitate An insoluble solid which may be formed on . 54K views 14 years ago Chemistry Demonstrations. The theoretical amount of MEA and NaOH to capture a ton of CO 2 is 1. Calcium hydride reacts with water to form calcium hydroxide and hydrogen gas.In fact, the CO 2 absorption capacity of NaOH solution is higher than that of MEA.Does solid sodium hydroxide react with carbon dioxide and air moisture? Ask Question. The overall CO (2) absorption reaction was carried out according to consecutive reaction steps that are generated in the order . In this Wikipedia article, it mentions: As the concentration of sodium carbonate increases, it undergoes hydrolysis to form sodium hydroxide.The reaction is: 2 NaOH (s) + CO2 (g) →Na2CO3 (aq) + H2O (l) This means that solid reagent grade sodium hydroxide is not pure enough to weigh and use directly.Glycerol was added at 0–89 wt% to achieve a liquid viscosity (μ L) of 0.How to Balance: NaOH + CO 2 → Na 2 CO 3 + H 2 O. Type of Chemical Reaction: For this . However, there is a decreasing reactivity .The general reaction is as follows: acid + base → water + salt.Balises :CO 2Carbon DioxideCarbon HydroxideSodium Carbonate 3: Chemical Reactions is shared under a license and was authored, remixed, and/or curated by LibreTexts. Therefore, to balance the sodium atoms we will consider $2$ moles of Sodium hydroxide to undergo this reaction., in which [H +] = [OH −] = 1.
JEE 2022: Chemistry- Reaction with Sodium Hydroxide
They are most quickly and easily represented by the equation: H+(aq) + OH− → H2O(l) (4) (4) H + ( a q) + O H − → H 2 O ( l) If you mix .

Balises :CO 2Sodium HydroxideReaction with Sodium Carbonate
REACTION OF SODIUM HYDROXIDE WITH CARBON DIOXIDE
$$ \ ce {CO2 + NaOH (aq) -> NaHCO3 (aq)} $$.For this, the absorption of diluted CO 2 (0. CO 2 absorption in NaOH solution has been studied extensively since the .REACTION OF SODIUM HYDROXIDE WITH CARBON DIOXIDE In this demonstration, a carbon dioxide (in the form of “dry ice”) is added to a solution of sodium hydroxide and .Reaction Information. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively.First, gaseous CO2 is physically absorbed (eq 1), which instantly reacts with a sodium hydroxide ion (OH−) to generate a bicarbonate ion (HCO3 −) (eq 2), that is further . 2 /day) plant using this process was already performed using the nternal i rate of return (IRR) and net present value (NPV) method [22].We review the currently available carbon dioxide absorbents: sodium hydroxide lime (=soda lime), barium hydroxide lime, potassium-hydroxide-free soda lime, calcium hydroxide lime and non-caustic lime. Pure water is a neutral solution A solution in which the total positive charge from all the cations is matched by an identical total negative charge from all the anions. It first neutralises the sodium hydroxide solution (causing the indicator to change from purple. Je voulais donc savoir quelle pouvait être la réaction entre l'hydroxyde de sodium et le dioxyde de carbone, et après des recherches, j'ai obtenu 2 réponses.Sodium Bicarbonate = Sodium Hydroxide + Carbon Dioxide.2 vol %) in carbonated NaOH solutions was experimentally studied in a temperature range relevant for direct air capture (DAC) in the middle Europe (5–20 °C).







