Solvent detergent plasma

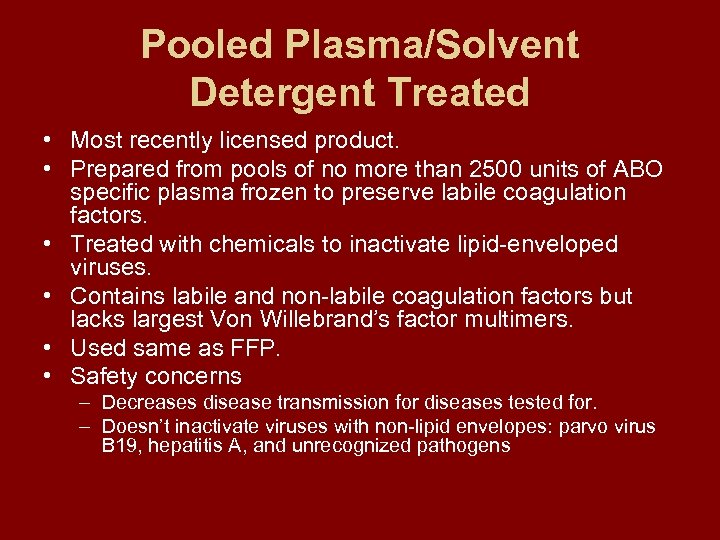
A preparation of solvent/detergent treated human plasma (frozen) from pooled donors is available from Octapharma ( OctaplasLG® ). This study evaluated the efficacy of solvent detergent treated plasma (S/D treated plasma) to reduce ATRs. OCTAPLASMA is solvent detergent (S/D) treated human plasma (45-70 mg/mL human plasma proteins).netTransition from fresh frozen plasma to solvent/detergent .Solvent/detergent-treated plasma was licensed >30 years ago. Study design and methods: Human plasma and immunoglobulin test materials were S/D-treated in Mobius single-use bags using 1% tri-n-butyl phosphate (TnBP) with 1% Triton X-100 or 1% Tween 80 at 31°C for 4 to 6 hours to evaluate the impact on protein .Solvent detergent-treated plasma (SDP) is a pathogen-inactivated blood plasma, which in comparison to frozen plasma is associated with lower rates of allergic reaction, transfusion-associated lung injury, and viral transmission.Solvent/detergent plasma is a pharmaceutical product with standardised content of clot-ting factors, devoid of antibodies implicated in transfusion-related acute lung injury . COVID-19 information.
Study design and methods: This .Octaplasma (plasma traité au solvant-détergent) : informations cliniques.OctaplasLG is a solvent/detergent (S/D) treated coagulation active plasma product, manufactured at Octapharma AB (Stockholm, Sweden), that contains all of the clotting factors and anticoagulants found in circulating human plasma.1 The pooled plasma is treated with a combination of 1% tri (n-butyl)-phosphate (TNBP) and 1% octoxynol. The introduction of a specific prion-binding ligand gel in combination with SD treatment, time-reduced from 4 to 1–1.Background: OctaplasLG is a frozen solvent/detergent-treated plasma product used for treating complex coagulation factor deficiencies or as substitution therapy in emergency situations where .
Solvent/detergent plasma: pharmaceutical characteristics and
Solvent detergent plasma is a form of blood plasma made from plasma collected from many people which is then processed with solvents as a form of virus processing, to try . Tor-Einar Svae, Octapharma AG, Lachen, Switzerland. Collisions et composition d’un plasma.govRecommandé pour vous en fonction de ce qui est populaire • Avis
FAQ : Plasma traité au solvant-détergent (Octaplasma)
Many parts of this guideline could also be applicable to active substances extracted from cellular .BACKGROUND: Solvent/detergent (S/D) treatment is an established virus inactivation technology that has been applied in the manufacture of medicinal products derived from human plasma for more than 20 years.Octaplasma S/D pathogen reduction is accomplished by pooling 630–1520 individual plasma donations. Andrea Heger, Octapharma Pharmazeutika Produktions GmbH, Vienna, Austria.Plasma cleaning has the capabilities to effectively remove all organic contaminations from surfaces through the process of a chemical reaction (air plasma) or physical ablation (Ar .Fresh frozen plasma (FFP) is prepared in blood banks world-wide as a by-product of red blood cell concentrate preparation. Search for more papers by this author.
[Product Monograph Template

Présentation PowerPoint narrée par le D r Johnathan Mack, médecin-conseil, Société canadienne du sang. Mast AE , Stadanlick JE , Lockett JM , Dietzen DJ.Solvent/detergent plasma is a pharmaceutical product with standardised content of clotting factors, devoid of antibodies implicated in transfusion-related acute lung injury . Recent reports on adverse events associated with solvent/detergent-treated plasma have raised concerns about the efficacy and safety of this type of therapeutic plasma.Pooled Plasma (Human), Solvent/Detergent Treated Solution for Intravenous Infusion.Donating blood.
Solvent detergent plasma
Octaplas® is human plasma for infusion of pharmaceutical grade with standardized .
Background Links. Blood, (11):3922-3927 1999. Donor centre hours & locations.
Manquant :
solvent detergent Les modes de génération d’un plasma.Plasma cleaning is the removal of impurities and contaminants from surfaces through the use of an energetic plasma or dielectric barrier discharge (DBD) plasma created from . Data on SDP use in Canada remains limited.Octaplasma is a pooled human plasma formulation that is pathogen inactivated through solvent detergent (S/D) treatment.Solvent detergent (S/D) treated plasma (Octaplasma)
(PDF) New Freeze-Dried Plasma as a Treatment Option in . The S/D additives are removed using castor oil and solid phase extraction.Auteur : Giancarlo Maria Liumbruno, Massimo Franchini
Octaplasma
Background: Solvent/detergent (S/D) inactivates enveloped viruses in plasma.This NAC Framework for Appropriate Use and Distribution of S/D Plasma provides an update to the 2015 NAC framework document.04-07 novembre 2019.
Frontiers
Appropriate clinical use is for coagulation factor disorders where appropriate concentrates are unavailable and when multiple coagulation factor deficits occur such as in surgery.
Solvent Detergent Plasma (SDP)
The line extension, which will be marketed in Europe as a powder and .Solvent detergent-treated plasma (SDP) is a pathogen-inactivated blood plasma, which in comparison to frozen plasma is associated with lower rates of allergic .Solvent Detergent Plasma. The solvent/detergent treatment is an established virus inactivation technology that has been industrially applied for manufacturing plasma derived medicinal products for . At the request of the PTBLC, CADTH completed two evidence‐based literature reviews in 2017 related to the use of S/D plasma. Important update to Octaplasma® availability Effective March 27, 2023, . Akute TTP-Episoden von 8 Patienten (Durchschnittsalter 27 Jahre, Bereich 18-44 Jahre) wurden im Hinblick auf die Verträglichkeit und die Wirksamkeit des verwendeten Plasmas . Study design and methods: All TTP patients who presented . Sign language interpreting services for Deaf, deafened and hard of hearing donors. The S/D treatment involves thawing and pooling . It has several specific characteristics, the most important being the standardized content of clotting factors, the lack of antibodies implicated in transfusion-related acute lung injury pathogenesis and the very high level of safety against transfusion-related viral infections.For fresh frozen plasma. Safety measures present .Octaplasma® is Solvent Detergent (S/D) treated, pathogen inactivated, standardized human plasma.Solvent/detergent-treated plasma (SD-plasma) has been developed in order to reduce the risks associated with the use of untreated FFP 8. Many parts of this guideline could also be applicable to active substances extracted from cellular components such as haemoglobin. Recognition program.Solvent/detergent-treated plasma has decreased antitrypsin activity and absent antiplasmin activity.The standardised content is achieved by pooling between 630 and 1520 single plasma units from multiple carefully screened donors. The current technology requires a plasma fractionation facility and is applied to large plasma pools, which increases the cost and risks of exposure to S/D-resistant pathogens and lowers the content of protein S and alpha2-antiplasmin. This pilot study was conducted to determine whether Thawed Solvent/Detergent-treated Plasma stored refrigerated for up to 5-days post-thaw (T-S/D) was as efficacious as TP.

Octapharma AG today announced that European medical authorities have approved the lyophilised presentation of the well-established octaplasLG® - pharmaceutically-licensed S/D treated plasma for transfusion.OctaplasLG is a frozen solvent/detergent-treated plasma product used for treating complex coagulation factor deficiencies or as substitution therapy in emergency .S/D Plasma is made from large pools of plasma that undergo pathogen inactivation using a solvent-detergent process and is then divided into 200 mL units. It is considered an environmentally safe process as it . Comparisons of various brands of solvent/detergent . The information on this page was selected from an Information Leaflet produced by the National Haemovigilance Office, issued in January 2003.Background: Thrombotic thrombocytopenic purpura (TTP) patients have increased risk for allergic transfusion reactions (ATR) due to the number of plasma products they require.
Guideline on plasma-derived medicinal products
Lothar Biesert, Octapharma Pharmazeutika Produktions GmbH, Frankfurt am Main, .Background: Octaplas is a solvent/detergent-treated, pooled plasma used for the management of preoperative or bleeding patients who require replacement of single or multiple coagulation .This study qualifies a single-use bag system for solvent/detergent (S/D) virus inactivation. Show 10 more references (10 of 26)Background: OctaplasLG is a frozen solvent/detergent-treated plasma product used for treating complex coagulation factor deficiencies or as substitution therapy in emergency situations where specific factor concentrates are not available.Abstract and Figures.Le mélange de plasma est traité avec une combinaison de tri (n-butyl)-phosphate (TNBP) 1 % et d’octoxynol 1 %. Solvent/detergent .
![[PDF] Efficacy and Safety Profile of Solvent/Detergent Plasma in the ...](https://i1.rgstatic.net/publication/45828233_Efficacy_and_Safety_Profile_of_SolventDetergent_Plasma_in_the_Treatment_of_Acute_Thrombotic_Thrombocytopenic_Purpura_A_Single-Center_Experience/links/00b7d52b84a07a6c87000000/largepreview.png)
1 OctaplasLG is therapeutically indicated for the treatment of complex coagulation factor deficiencies, .

Qu’est-ce qu’un plasma ? Fresh frozen plasma is prepared from the supernatant liquid obtained by centrifugation of one donation of whole blood. Les adjuvants (solvant et détergent) sont ensuite extraits à l’huile .Octaplas ® is a solvent/detergent (S/D) treated, pooled human plasma indicated for replacement of multiple coagulation factors in patients with acquired deficiencies due to liver disease or who are undergoing cardiac .

Solvent/Detergent Plasma.Lachen, Switzerland. Viral safety depends on donor . As a result of the .