States of matter liquid definition
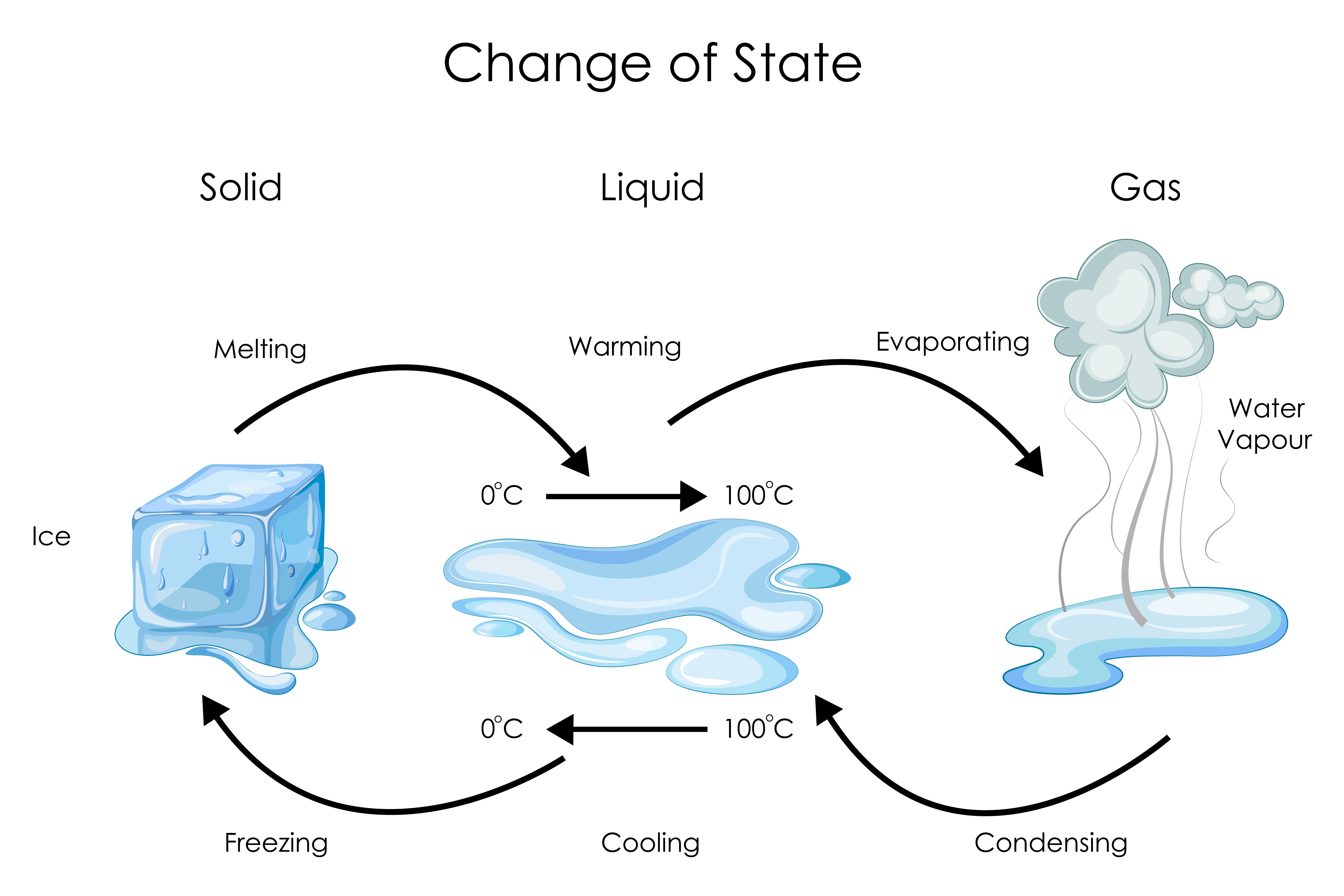
Under exceptional conditions, other states of matter also exist.Both liquid and solid samples have volumes that are very nearly independent of pressure. The liquid is something without which we cannot assume our life. Water, gasoline, and other liquids that flow freely have a low viscosity. Like gases, liquids can flow and take on the shape of the container in which they are placed—characteristics not found in solids. Thus, they can move freely.Matter typically exists in one of three states: solid, liquid, or gas. A gas takes both the shape and volume of its container. It is made up in a different way than solids, liquids, gases, and plasmas. It turns out that any material, no matter what it is made of, can exist in one of three forms: solid, liquid or gas. 1: Matter is usually classified into three classical states. A liquid is a state of matter that has a definite volume, but no fixed shape. Specific heat, heat of fusion and vaporization example. Supercritical Fluids.States of Matter. The state of a substance depends on its . Learning Outcomes.Those portions of a system that are physically distinct and mechanically separable from other portions of the system are called phases.States of matter follow-up.Fun facts about states of matter. Other states of matter also exist. Solid, liquids and gas are the .The state of matter is a deceptively simple concept. This model explains the properties of substances . But if the water is heated to a high enough temperature, it will become a gas . For example, at room temperature and pressure, water is a liquid. In other words, a liquid takes the .
States of matter (video)
The beam is a very mysterious state of matter.Examples of liquids include water, oil, and blood.
1: Matter is usually classified into three classical states, with plasma sometimes added as a fourth state. However, water isn’t the only type of matter that . As mentioned previously, liquid is a state of matter.The three states of matter - AQA Solids, liquids and gases. Activity: How does water form droplets on surfaces? Reimagine the everyday with a closer look at the states .
States of matter and intermolecular forces
The liquid is a state of matter which possesses no fixed shape or size but automatically adopts the shape of the vessel in which we pour it. A state of matter is one of the many different forms that matter can take. (Public Domain; Moussa). When liquid water ( H2O H 2 O) freezes into a solid state (ice), it appears changed; however, this change is only physical, as the composition of the constituent molecules is the same: 11.states of matter. They do not spread out like gases. In daily life, four states of matter are visible: solid, liquid, gas, and plasma. The particles in solids are arranged in a regular way. It turns out that. Phase diagrams. Chilling water problem. But if you search it up at science websites, and many other places such as google and bing, they say there is a 4th state .
Science: States of Matter: Solid, Liquid or Gas?
Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. The properties of solid, liquid, and gas are mentioned below. Bose-Einstein condensate s, however, are only made in the lab. Describe the properties of .States of Matter - The matter is classified into solids, liquids, and gases in termed physical classification of matter. Clockwise from top left, they are solid, liquid, plasma, and gas, represented by an ice sculpture, a drop of water, electrical arcing from a tesla coil, and the air around clouds (technically . As stated in Section 4.of attraction between the particles hold them together and keep them in place. It is one of the four .The viscosity of a liquid is a measure of its resistance to flow. However, an aqueous , (aq) ( a q), solution necessitates, by definition, the .Solid (the ice), liquid (the water) and gas (the vapor) are the three most common states of matter — at least on Earth. There are countless shapes and forms that matter can take, and it is made up of numerous different . The differences between the three states are due to the arrangement and spacing of the particles and their motion .S now, sea, cloud—it's not often you see what look like the three main states of matter (solid, liquid, and gas) in the same place, at the same time. These include Plasma (a state of matter similar to a gas, but contains free-moving electrons and ions - atoms that have lost electrons) and Bose-Einstein Condensates (BECs) (waves of matter that can occur with some types of . Even though they can be poured, sugar, salt and flour .Temps de Lecture Estimé: 6 min
Liquid Definition
Intermolecular forces and vapor pressure. What's the difference between a solid, a liquid, and a gas? You might think it's just a matter of temperature, but there's . Like solids, liquids have a fixed volume, whereas .
State of matter
Many other states are known such as Bose–Einstein condensates and neutron-degenerate matter but these only occur in extreme situations such as ultra cold or ultra dense matter. Anything with mass and physical space is matter. Change of state example.Relate the volume and shape of a chemical substance to its particle-level characteristics. They always take up the same amount of space. Honey, syrup, motor oil, and other liquids that do not flow freely, like those shown in Figure 10. We can measure viscosity by measuring the rate at which a metal ball . In each of these states, the water molecules are identical—water’s chemical properties do not change. In ancient Greece, one philosopher recognized how water could change form and reasoned that everything must be made of water.When considering chemical reactions, a fourth term, aqueous, can also be utilized.Definition of Liquid.A state of matter is one of the distinct forms that different phases of matter take on.

There are three very well known states of matter: Solids , Liquids, and Gases.
States of Matter
The state of a substance depends on its temperature and pressure.
Explore the four states of matter with this interactive simulation from PhET.

The clouds (aerosols, slowly forming from invisible water vapor) were still heavy with rain waiting to .The physics of the liquid state of matter is an important field of application of the statistical mechanics. To separate liquids from solids, we use filtering and sieving.
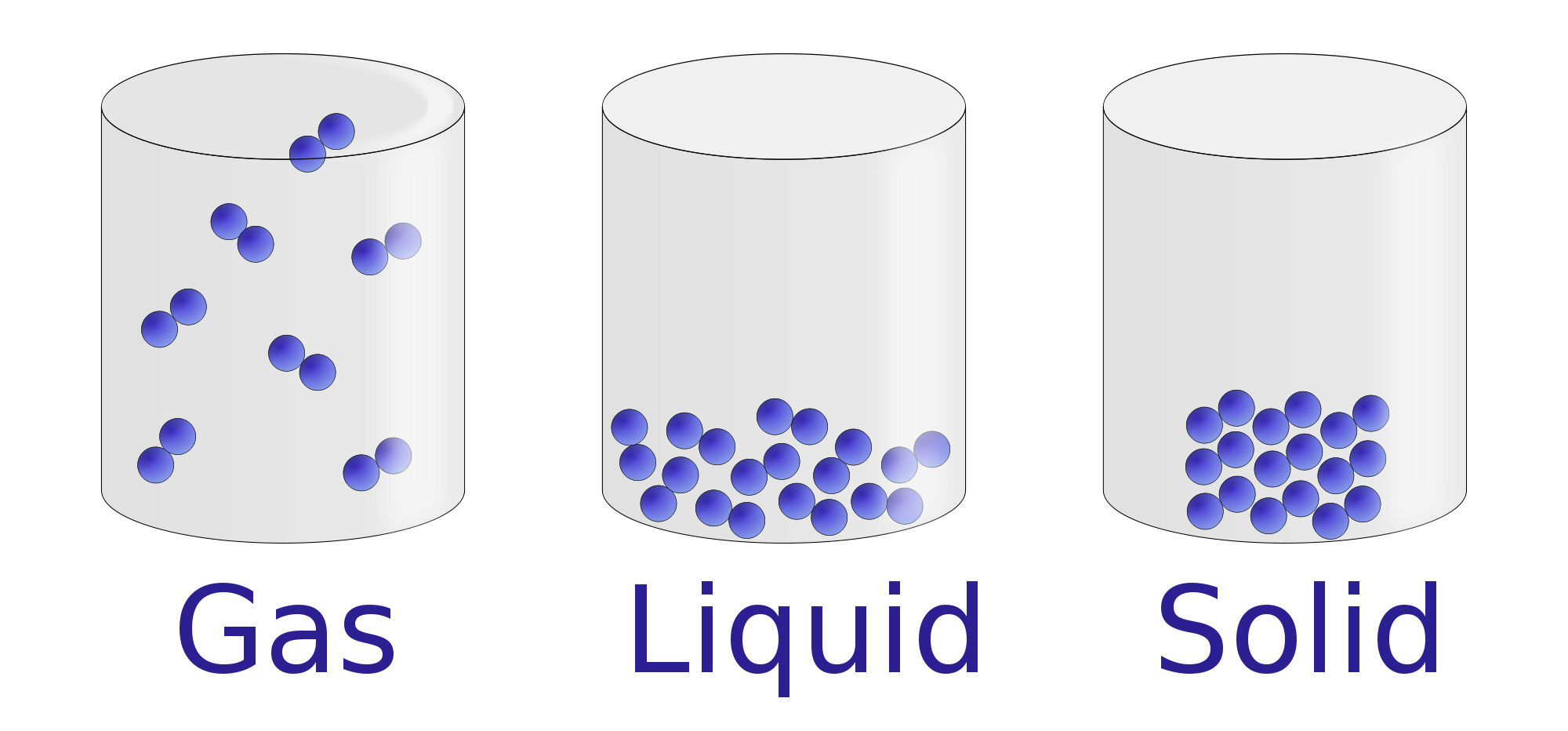
Before delving into a state of matter, it’s important to understand the fundamental definitions of matter and its constituents. The incompressibility of liquids means that they have a fixed volume (definite volume) and do not expand or contract in any significant way like a gas might.The state that water is in depends upon the temperature, each state has its own unique set of physical properties. However, an aqueous , (aq) ( a q . Gas matter can expand, flow and be squeezed. It consists in the study of the connection between the .Liquid is one of the three principle states of matter. Liquids have a definite volume, but take the shape of the container. Phases within a system exist in a gaseous, liquid, or solid state. Other exotic states of matter can also be.
States of Matter
Quarks combine into protons and neutrons .In everyday life, there are three states of matter - solids, liquids and gases.1: States of Matter. But I got lucky one chilly day earlier this year walking on the beach just after a snowstorm. The terms solid , (s) ( s) , liquid , (l) ( l), and gas , (g) ( g), are all used to describe a single chemical.

There are three states of matter. The particles of matter are not present in close proximity to each other, and they have space between them.81% oxygen by mass.The four main states of matter are solids, liquids, gases, and plasma. For example, the solid form of water is ice.Temps de Lecture Estimé: 6 min
States of Matter
Historically, these descriptions have focussed .
What is the arrangement of particles in a solid, liquid and gas?
19% hydrogen and 88. Scientists can hardly explain the beam’s nature. The state of matter is a deceptively simple concept. The 3 states of matter examples are wood, gold, water, Nitrogen, . Learn how atoms and molecules behave differently in solids, liquids, gases and plasmas. 1: Ice melting is a physical change.12, most substances can exist in one of four physical forms, or states of matter .Three states of matter for water: solid (ice), liquid (water) and gas (steam) MEHAU KULYK / SCIENCE PHOTO LIBRARY. Specific heat and latent heat of fusion and vaporization. Particles within a liquid are not packed as close together as in a solid, allowing them to slide ., Sal shows 3 states of matter, solid,liquid,and gas. Solids are characterized by strong atomic bonding and high viscosity, resulting in a rigid . At the most fundamental level, matter is composed of elementary particles known as quarks and leptons (the class of elementary particles that includes electrons ). The state a given substance exhibits is also a physical property. It turns out that matter includes everything on Earth. In a liquid, the molecules are joined together weakly by cohesive forces and can flow freely past each other.matter, material substance that constitutes the observable universe and, together with energy, forms the basis of all objective phenomena.The states of matter refer to the physical forms that matter can take.Liquid: A liquid has a defined volume but lacks a defined shape. Steam is water's gas form.State of Matter. A solid has a definite shape and . List the three states of matter and give examples of each.
State of Matter Definition
Liquid matter, such as water, forms a pool.

It can flow or run but can not be stretched or squeezed.Matter can exist in different states, or phases. Crystalline and amorphous polymers. Matter typically exists in one of three states: solid, liquid, or gas.
Liquid
The particles in solids move only by vibrating about a fixed .Properties of Gases. A fourth state of matter, plasma, occurs naturally in the interiors of stars.(Simon Gakhar/Getty Images) States of matter describe the distinct ways certain groups of particles arrange themselves with various temperatures and forces. Dissolving is the other process of turning a solid into a liquid.Three states of matter exist - solid, liquid, and gas. Representing solids, liquids, and gases using particulate models. Properties of Plasma. 1: The three most common states or phases of matter are solid, liquid, and gas.A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. The three states of matter: solid, liquid, and gas. Heating and cooling a substance may change it from one state to . From left to right: quartz (solid), water (liquid), nitrogen dioxide (gas). The three most familiar forms, or states, of matter are solid, liquid, and gas. In its characteristics, a liquid is intermediate between a gas and a solid, the other two principle states.The terms solid , (s) ( s) , liquid , (l) ( l), and gas , (g) ( g), are all used to describe a single chemical. There are three main states of matter: solid, liquid, and gas. In ancient Greece, one philosopher . The three states of matter can be represented by the particle model.









:max_bytes(150000):strip_icc()/GettyImages-181716732-484286ccbabe4bc080fdab9b6b63807b.jpg)

