Strontium hydroxide and hydroiodic acid

Hydrochloric acid = HCl.A strong acid has a pKa value less than -2.HC2H3O2 ⇌ H + (aq) + C2H3O − 2 (aq) As it turns out, there are very few strong acids, which are given in Table 11. Write a balanced chemical equation for the following reaction.Strontium oxide or strontia, SrO, is formed when strontium reacts with oxygen.Question: Enter formulas for the following neutral acids and bases. Beryllium hydroxide . Strong Acid + Strong Base B.
Strontium oxide Strontium peroxide: Other cations. By definition, a strong acid yields .Water is the base that reacts with the acid HA, A − is the conjugate base of the acid HA, and the hydronium ion is the conjugate acid of water. Those bases lying between .Strontium hydroxide, Sr(OH) 2, is a caustic alkali composed of one strontium ion and two hydroxide ions. The limited solubility of hydroxides is taken into account (as indicated by footnotes in the last column).Strontium hydroxide is considered a strong base because of its capacity to dissociate fully in aqueous solution to produce Sr 2+ and OH – ions.Be sure to specify states such as (aq) or (s). It is synthesized by combining a strontium salt with a strong . , it is a strong acid. Those bases lying between water and hydroxide ion accept protons from water, but a mixture of the hydroxide ion and the base results. The stem of the other element’s name, fluorine, is fluor, and we must also include the -ic acid ending.68 g of strontium sul. This compound’s basic nature . In this reaction, a base reacts with an acid to produce water and a salt.Auteur : Wayne Breslyn tetragonal (octahydrate) Hazards NFPA 704 (fire diamond) 1. This is achieved by including the corresponding solid phase into the equilibrium calculation.Water is the base that reacts with the acid HA HA, A− A − is the conjugate base of the acid HA, and the hydronium ion is the conjugate acid of water.0 x 10 8; hydrochloric: HCl: 1. d)strontium hydroxide.Regarder la vidéo1:5729. Strontium reacts slowly with water, forming strontium hydroxide, Sr(OH) 2 and hydrogen gas, H 2. As a binary acid, it belongs to the group of halogen-based compounds, sharing attributes with other hydrogen halides. Ionic Equation: Hydrobromic acid is soluble in water, meaning it will dissociate into its ions, but strontium hydroxide is insoluble. Similarly, Arrhenius defined a base as a compound that increases the concentration of hydroxide ion (OH −) in aqueous solution. molecular equation. Method 3500-Sr C Inductively Coupled Plasma Method [2].Strong Acid Dissociation Constants at 25°C.

hydrochloric acid.Regarder la vidéo0:58In this video we'll write the correct formula for Hydroiodic acid.

solid copper(I) hydroxide and hydroiodic acid e. This reaction is classified as. Write the balanced NET IONIC equation for the reaction that occurs when nitrous acid and potassium hydroxide are combined.Consider HCl(aq).Solution for Complete and balance the reaction between strontium hydroxide and hydroiodic acid.
pH of Common Acids and Bases
2H + (aq) + 2OH-(aq) ==> 2H2O(l) . This base is named as an ionic compound between the strontium ion and the hydroxide ion: strontium hydroxide.2 x 10 8; perchloric: HClO 4: 1. perchloric acid [HClO 4 (a q)] and solid aluminum hydroxide d.44 HNO 2: nitrous acid: 3.6: Acid–Base Titration is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. hydroiodic acid 3. Any acid that dissociates 100% into ions is called a strong acid. aqueous strontium hydroxide and hydrobromic acid. This covalent molecule of 1 H and 1 I forms a strong acid, typically in an aqueous solution. aqueous strontium hydroxide and . Enter formulas for the following neutral acids and bases. Bases that are weaker than water (those that lie above water in the column . The nitrogen in C 5 H 5 N would act as a proton acceptor and therefore can be considered a base, but because it does not contain an OH compound, it cannot be considered a strong base; it is a weak base. b)hydroiodic acid.HClO 4 perchloric acid: LiOH lithium hydroxide: HCl hydrochloric acid: NaOH sodium hydroxide: HBr hydrobromic acid: KOH potassium hydroxide: HI hydroiodic acid: . For example, hydrochloric acid has a pKa value of about -5. write formulas for the following acids and bases: a)barium hydroxide. Calcium hydroxide neutralizes perchloric acid.Write the balanced formula equation for the acid–base reactions that occur when the following are mixed.When aqueous strontium hydroxide (Sr(OH)2) and hydroiodic acid (HI) are mixed, a neutralization reaction occurs, which is a specific type of acid-base reaction.Chemistry questions and answers. Report Still looking for help? Get the right answer, fast. barium hydroxide (aqueous) and hydrochloric acid c. Enter an equation of an ionic chemical equation and press the Balance button.
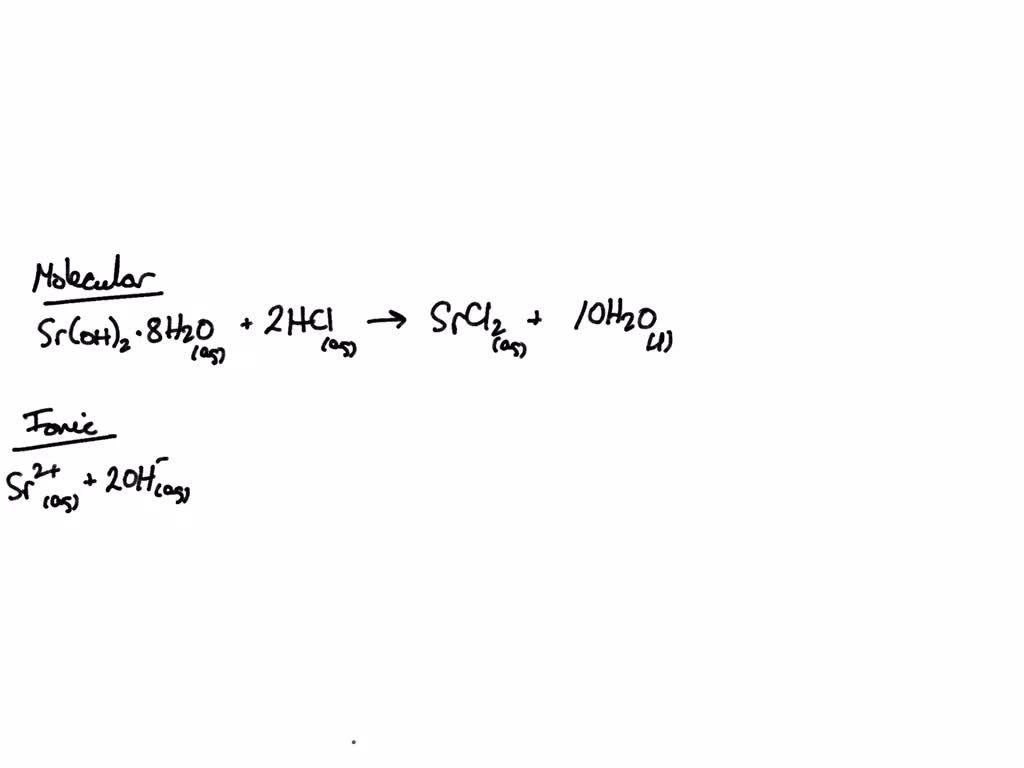
Solved Write the balanced formula equation for the acid-base
strontium hydroxide 5.Complete and balance the reaction between strontium hydroxide and hydroiodic acid. Ho NaOH H2SO4 acid and and.Chemically, hydroiodic acid is a simple compound comprised of one atom each of hydrogen (H) and iodine (I). Write the balanced molecular, ionic and net ionic equations. calcium hydroxide (aqueous) and hydrochloric acid c.6K views 3 years ago.3 (first OH –), 0.83 (second OH –) Magnetic susceptibility (χ) −40. sodium hydroxide (aqueous) and nitric acid b.The balanced formula equation for the acid-base reaction occurring when aqueous strontium hydroxide and hydroiodic acid are mixed is: Sr(OH)2(aq) + 2HI(aq) → SrI2(aq) + 2H2O(l) Here, we have strontium hydroxide, a strong base, reacting with hydroiodic acid, a strong acid. Its name is hydrofluoric acid. A portion of the sample is digested in a combination of acids.
Strontium: Chemical reactions
Strontium hydroxide = Sr(OH) 2. Sr (s) + 2 H 2 O (l) Sr(OH) 2 (aq) + H 2 (g) Quantitative analysis. By definition, a strong acid . When they react, they form strontium iodide and water, which is a .
Solved hydroiodic acid (aq) + strontium hydroxide (aq) →
45 g of strontium phosphate and 5. PDF Version [78. , it is a strong base. Solid silver hydroxide and hydroiodic acid Aqueous barium hydroxide and hydroiodic acid. Generally speaking, these values are not used in calculations since, at common concentrations in chemistry, each substance is 100% dissociated.The acid-base reaction of hydrobromic acid and strontium hydroxide is given as: {eq}\rm 2HBr(aq) + Sr(OH)_2(s) \to SrBr_2(aq) + 2H_2O (\ell) {/eq}.A strong base, such as one of those lying below hydroxide ion, accepts protons from water to yield 100% of the conjugate acid and hydroxide ion. If a box is not needed, leave it blank. The pKa value of the acid depends on the solvent.
Strontium oxide
Temps de Lecture Estimé: 8 min
Is Sr(OH)2, Strontium Hydroxide, an Acid, Base, or Neutral?
Burning strontium in air results in a mixture of strontium oxide and strontium nitride.2 x 10 9; hydrobromic: HBr: 1. When these react, they produce liquid water and strontium . lead (II) nitrate (aq) + sodium iodide (aq) to lead (II) iodide (s) + sodium nitrate (aq); For example, hydrochloric acid (\(\ce{HCl}\)) is an acid because it . It occurs as an anhydrate whose prismatic, colorless crystals are . W; Provide the net ionic equation for the reaction that occurs when aqueous solutions of potassium hydroxide and hydroiodic acid are combined. Predict whether nickel(II) .13 HNO 3: nitric acid: 3.Write the balanced formula equation and net ionic equation for the acid-base reactions that occur when the following are mixed: aqueous strontium hydroxide and hydroiodic acid.Strontium hydroxide has the molecular formula of Sr (OH)2 and the molecular weight of 121.3 KB] This Public Health Statement is the summary chapter from the Toxicological Profile for strontium.0·10 −6 cm 3 /mol Structure Crystal structure. Because Mg (OH) 2 is listed in Table 9.solid strontium hydroxide with hydrobromic acid aqueous sulfuric acid with solid sodium hydroxide A neutralization reaction gives calcium nitrate as one of the two products. The earliest definition of acids and bases is Arrhenius's definition which states that: An acid is a substance that forms hydrogen ions H + when dissolved in water, and; A base is a substance that forms hydroxide ions OH-when dissolved in water. One of the standard laboratory exercises in General .Strontium dissolves readily hydrochloric acid, forming Sr(II) . There are 2 steps to . It may be 1% ionized or 99% ionized, but it is still classified as a weak acid.Sodium hydroxide: NaOH Potassium hydroxide: KOH Rubidium hydroxide: RbOH Cesium hydroxide: CsOH Calcium hydroxide: Ca(OH) 2; Strontium hydroxide: .0 x 10 9; perbromic: HBrO 4: 3. net ionic equation.
Relative Strengths of Acids and Bases
Flash point: Non-flammable Related compounds Other anions. perchloric acid. potassium hydroxide (aqueous) and nitric acid b. A) Write a balanced equation for the reaction. reaction of aqueous strontium hydroxide with aqueous hydroiodic acid5.The total ionic equation and the net ionic equation for a chemical reaction Consider the neutralization reaction between potassium carbonate, K2CO3, and hydrobromic acid, HBr, forming the salt potassium bromide, KBr, .
Strong Acids and Bases
barium hydroxide 2. perchloric acid $\left[\mathrm{HClO}_{4}(a q)\right]$ and solid iron(III) hydroxide d.50 g of sulfuric acid are used and 5.This acid has only two elements in its formula, so its name includes the hydro- prefix.Strontium phosphate reacts with sulfuric acid, H2SO4, to form strontium sulfate and phosphoric acid, H3PO4.All neutralization reactions of a strong acid with a strong base simplify to the net ionic reaction of hydrogen ion combining with hydroxide ion to produce water.Strontium hydroxide (aqueous) and hydrobromic acid Perchloric acid [HClO4 (aq)] and solid iron(III) hydroxide d.Funded by Saskatchewan Educational Technology Consortium. Acid-base titrations are lab procedures used to determine the concentration of a solution. It is one in a series of Public Health . Hydroxides are strong bases but have low solubility which confines the pH to an upper bound. One of the simpler acid base theories states that acids donate H+ ions and bases donate OH- ions. When HCl is dissolved in H 2O, it completely dissociates (separates) into H + (aq) and Cl − (aq) ions; all the HCl molecules become ions: HCl 100% → H + (aq) + .Arrhenius defined an acid as a compound that increases the concentration of hydrogen ion (H +) in aqueous solution. f)hypochlorous acid. Upvote • 1 Downvote Add comment More.pH of Common Acids and Bases. HNO 3 nitric acid : H 2 SO 4 sulfuric acid: HBr hydrobromic acid: HI hydroiodic acid: HClO 3 chloric acid: HClO 4 perchloric acid The 8 Strong Bases.hydroiodic acid: 3.soluble in acid, NH 4 Cl: Basicity (pK b) 0. Acid Name: Formula: K a; hydroiodic: HI: 3. 2- Write the formula for each of the following acids and bases: a) magnesium hydroxide b) hydrofluoric acid c) phosphoric acid d) lithium hydroxide e) ammonium hydroxide f) . If an acid is not listed here, it is a weak acid.