Sunshine act definition

The Pennsylvania Sunshine Act: A Comprehensive Overview
En France, consacrées par .
Everything you need to know about the Sunshine Act
The UK is falling behind as countries around the world introduce legislation to increase the transparency of payments made from the pharmaceutical industry to healthcare professionals.
The Government in the Sunshine Act: An Overview
It was passed into law in 2010 as part of the Affordable Care Act.
16 CFR Part 1013
What is meant by research for the purposes of the Sunshine Act? federal healthcare programs to report payments, transfers, ownership and items of value given to physicians and .Where the charges are calculated to be an amount in excess of $25.AppZen is the world’s leading automated solution for Sunshine Act compliance.00 and the calculated charges.
Physician Payments Sunshine Act — Wikipédia
Various European countries also have sunshine payment acts in force.Partager par mail.
Open Payments Law and Policy
one of ten exemptions.2 The Act reflects the feeling that greater. 41, §1, 3°, Sunshine Act): o professionnel du secteur de la santé (voir plus loin) o organisation du secteur de la santé (voir plus loin) o organisation de patients (voir plus loin).Click here to consult the text of the Royal Decree of 14 June 2017 executing the Sunshine Act, Belgian official Journal 23 June 2017 Royal Decrees of recognition Click here to consult the text of the Royal Decree d.SUNSHINE ACT 1.
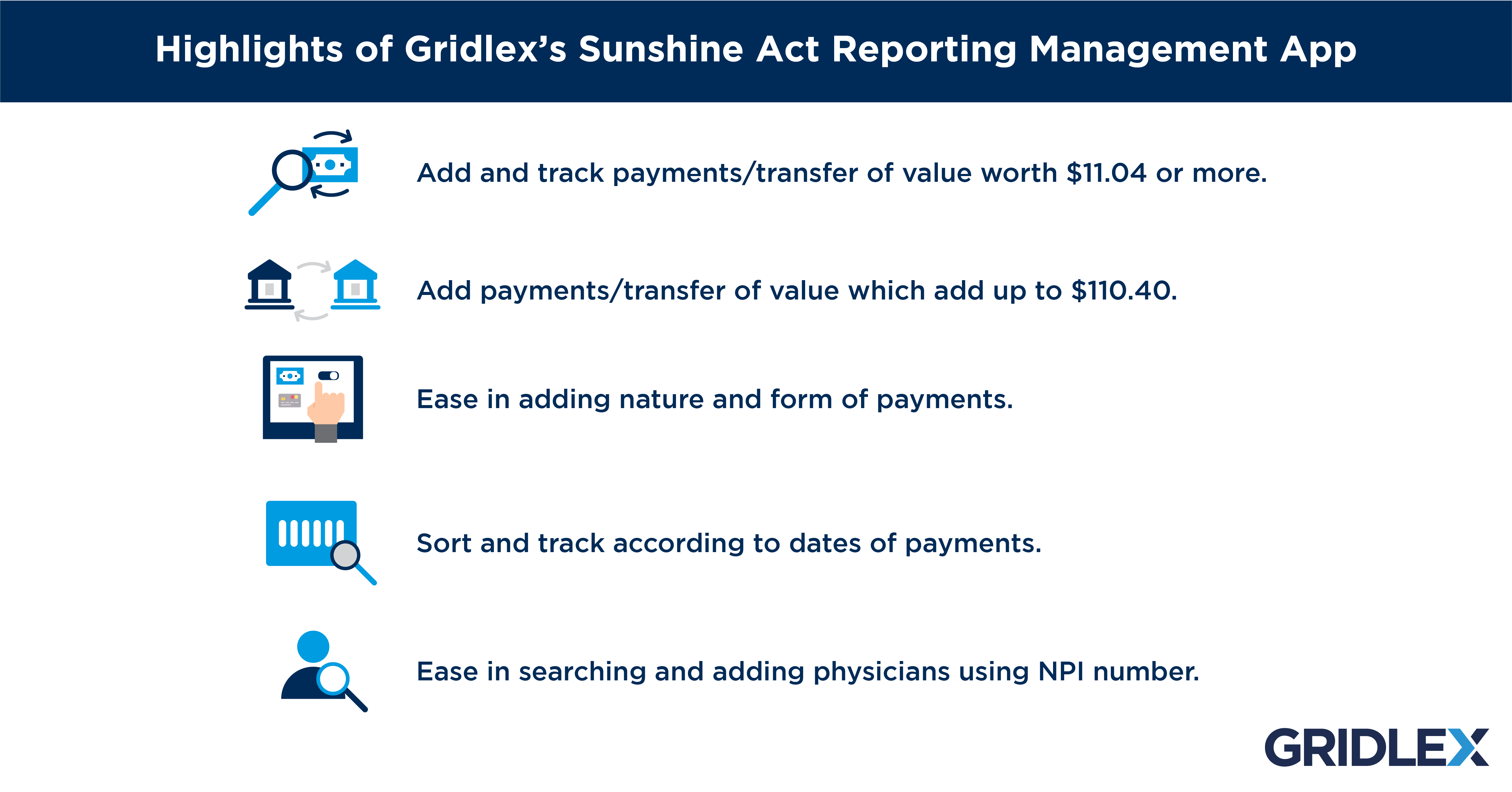
Open Payments houses a publicly accessible database of payments that reporting entities, including drug and medical device companies, make to covered recipients like physicians.Le décret relatif au « Sunshine Act à la française » a été publié au « Journal officiel » du 22 mai. La loi impose la transparence des liens entre les industries de santé et les autres acteurs du monde de la santé (professionnels de santé, étudiants, .By Natalie Rhodes.Key Provisions of the Pennsylvania Sunshine Act Definition of a Meeting The act defines a meeting as any prearranged gathering of a quorum of members of an agency where deliberation or official action takes place. International comparison.2021 which mandates once again the Mdeon vzw-asbl to manage the platform betransparent.
Government in the Sunshine Act
Source : infirmiers.Physician Payment Sunshine Act. The Office of Open Records (OOR . This definition does not include distributors .The Sunshine Act and accompanying regulations largely, but not entirely, preempt state and local laws and regulations.On April 23, 2024, the Federal Trade Commission (FTC), in a 3-2 vote, issued a final rule that bans noncompete clauses between workers and employers as “unfair .

Le décret baptisé “Sunshine Act“ (en référence à la loi américaine visant à garantir l’indépendance des experts et la déclaration des liens d’intérêt), crée une .
Manquant :
sunshine actFederal Register :: Sunshine Act Meetings
Sunshine laws make meetings, records, votes, deliberations and other official actions available for public observation .Le Sunshine Act exige que tout fabricant de médicaments, de dispositifs, de matériels médicaux ou biologiques, ainsi que tout groupement d’achats de ces mêmes produits, déclare tout paiement, en numéraire ou en nature, supérieur à 10 dollars, consenti à un médecin ou à un centre hospitalier universitaire.
A sunshine payment act for the UK
Section 703 of the Act defines “agency” as: “The body, and all committees thereof authorized by the body to take official action or render advice on matters of agency business, of all the following: the General Assembly, the executive branch of the .Quelques définitions dans le cadre de l’obligation de transparence. b) RD Sunshine Act: Royal Decree of 14 June 2017 executing the Sunshine Act, Belgian official Journal 23 June 2017.The Sunshine Act is a federal law that requires manufacturers of covered drugs, devices, biologics or medical supplies to collect detailed information about .

This act compels pharmaceutical and medical device manufacturers to declare transfers of value (including expenses) to physicians or teaching hospitals. The Physician Payments Sunshine Act is a 2010 United States . La « Sunshine Act » correspond à la « Physician Payments Sunshine Act » (il s’agit de la section 6002 de l’Affordable Care Act de 2010), .
Manquant :
definitionFREQUENTLY ASKED QUESTIONS SUNSHINE ACT
The act requires manufacturers of drugs, medical devices and biological, and group purchasing organizations (GPOs) that participate in U.The Physician Payments Sunshine Act is a disclosure law requiring all drug, medical device, and biologics companies to report transfers of value to physicians and teaching hospitals.If you are a physician working in the United States, 1 August marks a watershed moment.
FREQUENTLY ASKED QUESTIONS ABOUT THE SUNSHINE ACT
Physician Payments Sunshine Act.January 1, 2021.
Manquant :
definition
One year on from First Do No Harm, the British government has accepted a similar requirement in principle.Sunshine Act: la transparence dans l’intérêt du patient. Employees no longer have to shoulder the load of documenting every single meeting with a medical professional or depend on 3rd-party databases for validation. Importantly, the act considers any communication or exchange of information relating to agency business as part of the .BE: la transparence des collaborations entre les .In response to a continuing concern that the federal government.Le Physician Payments Sunshine Act (“Sunshine Act”), qui fait partie du Affordable Care Act de 2010 (« ACA ») est une loi fédérale américaine entrée en vigueur pour faciliter la transparence des .
« Sunshine Act à la française
Sunshine Act: la transparence dans l’intérêt du patient
g) Bénéficiaire (art.
Manquant :
definitionLe Sunshine Act
Thus, manufacturers and recipients must determine whether any additional laws or regulations are relevant to specific situations.Individuals who plan to attend in person and who require special assistance, such as sign language interpretation or other reasonable accommodations, .
Manquant :
definition La loi Sunshine Act est entrée en vigueur en . federal health care programs to report certain payments and items of value given to physicians and teaching hospitals.Auteur : Magali Guegan, Maurice-Pierre Planel The preamble to the original 2013 rule implementing the Sunshine Act affirmed that PODs are a form of group purchasing organization (“GPO”) subject to the law’s reporting requirements, but did not define the term “physician owned distributor.The Sunshine Act is intended to make relationships between certain pharmaceutical and device manufacturers and healthcare providers more transparent, by requiring .The Open Payments program is a national disclosure program that promotes a more transparent and accountable health care system.Section 6002 of the Act, entitled “Transparency Reports and Reporting of Physician Ownership or Investment Interests,” is commonly known as the “Sunshine .Le Physician Payments Sunshine Act ou communément Sunshine Act , est une loi américaine datant de 2010 prise dans le domaine de la santé publique et visant à . the Sunshine Act' in 1976, requiring that all portions of all. accountable to the American people, Congress enacted the Government. Only four of the nine DFC board members are appointed by the President with the advice .While the act generally preempts existing state laws where they require reporting the same information by the same entities, the scope of exceptions to this preemption is unclear, and the act does . AppZen’s AI examines every business attendee to determine if he or she is likely to be a medical .L’objectif de cette loi Sunshine Act (et des lois « sunshine » similaires) est de permettre aux patients de prendre connaissance des relations financières entre les professionnels de santé, les médecins et les hôpitaux universitaires et les fabricants et distributeurs de produits médicaux.00, the fee charged shall be the difference between $25. Article publié 26-07-17.Le « Physician Payments Sunshine Act » adopté en mars 2010 aux Etats-Unis dans le cadre de la réforme de la santé menée par Barack Obama (Obamacare), vise à obliger . The first set of data was released via an online public database on September 30, 2014, with . Some definitions in the context of the transparency obligation a) Sunshine Act: chapter 1 of title 3 of the Law of 18 December 2016 regarding various provisions on health, Belgian official Journal 27 December 2016.Manquant :
definitionSunshine Act » actif depuis le 1er octobre 2013
En quoi consiste exactement l’obligation de transparence? L’obligation de transparence impose entre autres aux entreprises pharmaceutiques et .Le « Physician Payments Sunshine Act » adopté en mars 2010 aux Etats-Unis dans le cadre de la réforme de la santé menée par Barack Obama (Obamacare), vise à obliger les laboratoires pharmaceutiques et entreprises de dispositifs médicaux à déclarer auprès du Département américain de la Santé, toute somme ou tout cadeau excédant 10 . The ability of the pharmaceutical industry to influence healthcare policy and decision making through gifts and payments is . For most journals, authors must disclose conflicts of interest and funding support. Only transactions of $10 or more (with .The Sunshine Act was introduced originally to bring greater transparency to payments made by pharmaceutical and medical device companies to physicians and . 111-148) Health Care and Education Reconciliation Act of 2010 (Public Law No. conducted by federal agencies be open to the public unless they fit.The Sunshine Act applies only to agencies “headed by a collegial body composed of two or more individual members, a majority of whom are appointed to such position by the President with the advice and consent of the Senate. This is when the Physician Payments Sunshine Act, which is part of .
Manquant :
definitionSunshine Act à la française : l'opération transparence des labos
Adopté aux États-Unis en 2010 et connu sous le nom de Physician Payments Sunshine Provisions ou de Physician Sunshine Act, ce dispositif est un des volets d’une série de .The Pennsylvania Sunshine Act, 65 Pa.intermédiaire (voir aussi la définition de « l’année de référence »). 111-152) SUPPORT for Patients and Communities Act (Public Law No. The Physician Payments Sunshine Act (Sunshine Act), which is part of the Affordable Care Act (ACA), requires manufacturers of drugs, medical devices, and biologicals that participate in U.