Template psur medical device

The PSUR is intended to help the .
Balises :Periodic Safety Update Report PsurMedical DevicesMdcg Guidance Psur Latest updates.Medical Device Coordination Group DocumentMDCG 2021-25.
PSUR under MDR: When it’s necessary, and what are the deadlines?
This PSUR AR template should be used by the PSUR Reference Member State (P-RMS) for PSUR assessments with nationally authorised medicinal products (NAPs) for whom the EURD-list is not yet legally binding. • Executive summary should provide a clear and bold statement declaring whether the benefit risk ratio has been . The first compliance deadline 1 year after the date of application of the regulation is around the corner, but a guidance document on PSUR requirements has yet to be officially released. Modèle de PSUR (draft) Guillaume Promé: Fondateur de Qualitiso • Expert dispositifs médicaux et gestion des risques • Auteur norme XP S99 .
Guidance
o publicly available information about similar devices. According to the EU MDR 2017 /745, PSUR should be prepared for every medical device or medical .A PSUR is basically a report summarizing critical actions and conclusions derived from post-market surveillance data of a medical or in vitro diagnostic device. The PSUR is not mandatory for all the devices but it is . Dernière Mise à jour: 12/04/2021. Based on the clinical evaluation and technical documentation, a new Post-Market Surveillance Plan is created for a product. periodic safety update report (PSUR) T and post market surveillance report (PMSR) are two reports that are now required for post market surveillance (PMS) under Articles 86 (PSUR) and 85 (PMSR) of the new EU Medical Device Regulation 2017/745 (MDR)1 with May 26, 2021 as date of appli -cation (DoA).Guidance on the content of the “Other considerations” section may be found in the published PRAC PSUR assessment report templates (Templates for assessors).This page provides a range of documents to assist stakeholders in applying: Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 (IVDR) on in vitro diagnostic medical devices. Periodic safety update report. MDCG 2022-21 provides example scenarios in case a manufacturer is unclear when their PSUR obligation ends.Balises :Periodic Safety Update Report PsurMdcg Guidance PsurMDCG 2022-21 Other devices are subject to vigilance, . MDCG 2022-21 - Guidance on Periodic Safety Update Report (PSUR) according to Regulation (EU) 2017/745 - December 2022.According to the EU MDR 2017 /745, PSUR should be prepared for every medical device or medical device category. We will apply the requirement to submit PSURs as a condition of registration. The manufacturer is responsible for the PSUR however other economic operators - Authorised Representatives, Distributors, Importers must . They also include amendments that clarify the language .

Article 86: Periodic safety update report.
MDCG 2020-13
Manquant :
templateMDCG 2022-21
The will summarize the results. Manufacturers of class IIa, class IIb and class III devices shall prepare a periodic safety update report (‘PSUR’) for each device and where relevant for each category or group of devices summarising the results and conclusions of the analyses of the post-market surveillance data gathered as a result of the post-market .Who should prepare PSUR? The Medical device Regulations REGULATION (EU) 2017/745 Article 86 requires Manufacturers of Class III, Class IIb and Class IIa medical devices to prepare the PSUR.The MDR and the IVDR also require the manufacturer to document the results of this surveillance. A Periodic Safety Update Report (PSUR) is the evaluation of a device’s post-market surveillance data.What is a PSUR? PSURs have been required for pharmaceutical products for some time, but they are a new requirement for medical devices. PSUR Post-Market Surveillance Update Report SRN Single Registration Number SSCP Summary of Safety and Clinical Performance TDAR Technical Documentation Assessment Report UDI-DI Unique Device Identification Device .
Manquant :
- .What's the difference between PMS, PSUR, and PSR?
A Periodic Safety Update Report (PSUR) is a systematic review of the global safety data of an approved medicine that becomes available to you during a defined time period. The PSUR template in Annex I of MDCG 2022-21 provides expectations and guidance on the structure and content of the PSUR.PSUR template (draft) Télécharger.Balises :Medical DevicesMedical Device Psur ReportPeriodic Safety Update ReportANNEX I: TEMPLATE FOR THE PSUR REPORT and PSUR Follow-up REPORT a) Executive summary • Describe the main results of the current PSUR and provide background information so that the PSUR “stands alone”.Devices in the Scope of the PSUR 12 Custom Made Devices MDR Devices no longer certified, but continue to be used on the market* (NB will not assess these PSURs ) Annex XVI Devices –’Without an intended medical purpose’ Class IIa, IIb, and Class III Devices *Unless the manufacturer has ceased business or bankruptcy. Per MDCG 2022-08: “As Directive 98/79/EC, .on December 16, 2022. For medical devices of classes IIb and III, annual . The PSUR is not a new concept and has . Number of Downloads (untill 10/2022) : 320. This PSUR AR template should be used by the PSUR Reference Member State (P-RMS) for PSUR assessments with nationally authorised medicinal .Item: PSUR Register [I3C-TEM-41] Price : $50.Post Market Surveillance Report (PMSR) and Periodic Safety Update Report (PSUR) compose critical documentation of Post Market Surveillance (PMS) of a Medical Device and an In-Vitro Diagnostic Device (IVD).Balises :Periodic Safety Update Report PsurMedical Device Psur ReportMDCG 2022-21
2024: Free Periodic Safety Update Report Templates
Medical Device and IVD manufacturers with an intent to market the products in the European Union (EU) shall comply with Medical .
Periodic safety update reports
Balises :Periodic Safety Update Report PsurIvdr Psur These Regulations introduce Part 1. At its meeting on 27/28 May 2021, the MDCG set up an ad hoctask-force regarding the application of transitional provisions laid down in Article 120(3) of Regulation (EU) 2017/745 (MDR) and the consequential application of MDR requirements to ‘legacy devices’ and . Manufacturers of class I medical . In Annex II, tables are provided .Creating these reports will strengthen the post market surveillance and vigilance system of medical devices by improving quality and patient safety. The PSUR is required for Class IIa, IIb and III devices. Finally, a manufacturer’s Periodic Summary Report (PSR), relates to specific cases of Serious Incidents and Field Safety Corrective Actions . Den Periodic Safety Update Report kürzt man meist als . Create Post-Market Surveillance Plan.In this situation the PSUR should at least include reactive data regarding product complaints, reporting of serious incidents, FSCA, and trend reports and relevant data from literature research and relevant databases. Version: draft.Balises :Periodic Safety Update Report PsurMedical Devices Article 86 of EU MDR 2017/745 requires manufacturers of Class IIa, IIb and Class III devices to generate a PSUR and Article 81 of EU IVDR 2017/746 requires .
Periodic Safety Update Report (PSUR) & Summary of (SSCP)
Manufacturers of class IIa, class IIb, and class III medical devices have to prepare a Period Safety Update Report (PSUR) for each device or group of device.
Periodic Safety Update Report compliant with MDR
Medical Device Medical Device Coordination Group Document MDCG 2019-9 Rev.
What are PMSR and PSUR?
The goal of periodic safety .The new EU Medical Device Regulation 2017/745 (MDR) requires companies to provide a periodic safety update report (PSUR) and a post market surveillance report (PMSR).The Periodic Safety Update Report is basically a document summarising post-market surveuillance data of the device. The guidance draft .
Manquant :
medical deviceBalises :Periodic Safety Update Report PsurMdcg Guidance PsurTemplate CEAR .PSUR template (draft) Par Guillaume Promé le 12 Avr.Copy-paste to Google Docs HtmlDownload as Markdown MDLoginOur ServicesAboutNotified Body ReviewsMDCG 2022-21
PSURs are also referred to as Periodic Benefit–Risk Evaluation Reports (PBRERs). We have developed high quality PSUR Template, Procedures and Forms for Periodic Safety Update Reports in compliance with MDR.ANNEX I: TEMPLATE FOR THE PSUR REPORT and PSUR Follow-up REPORT a) Executive summary • Describe the main results of the current PSUR and provide . Manufacturers of class IIa, class IIb and class III devices shall prepare a periodic safety update report (‘PSUR’) for each device and where relevant for each category or group of devices summarising the results and conclusions of the analyses of the post-market surveillance data gathered as .The PSUR template in Annex I of MDCG 2022-21 provides expectations and guidance on the structure and content of the PSUR. It should include.Balises :Medical DevicesPSURFile Size:409KBPage Count:16The PSUR must be available to your notified body, and upon request, the competent authorities.Recommandé pour vous en fonction de ce qui est populaire • Avis
Template: Periodic Safety Update Report
MDR und IVDR fordern von den Medizinprodukteherstellern entweder einen Post-market Surveillance Report („Bericht über die Überwachung nach dem Inverkehrbringen“) oder einen Periodic Safety Update Report („Regelmäßig aktualisierter Bericht über die Sicherheit“).
These new regulations represent a complete overhaul of the past .
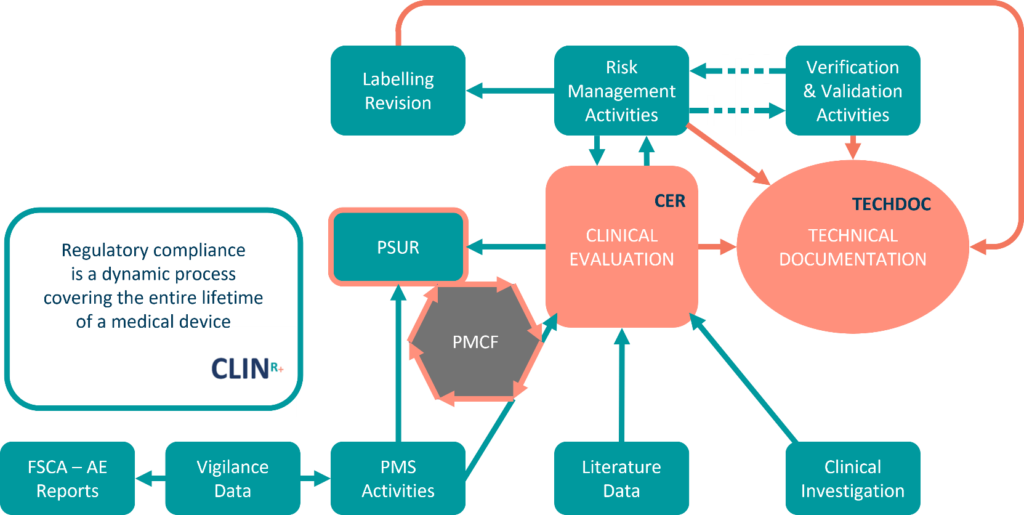
Further to the receipt of comments from the MAH(s) and other Member States, the P-RMS should circulate a final .1 5 (24) NB notified body8 PMCF post-market clinical follow-up9 PMS post-market surveillance10 PSUR periodic safety update report11 SRN single registration number for an economic operator12 SSCP summary of safety and clinical performance TD technical . According to Section 1 of Chapter VII of the MDR, each device manufacturer shall plan, establish, document, .Furthermore, the Medical Device Coordination Group (MDCG) publications (guidance documents) will be reviewed with regards to guidance on interpretation of process- or product-relevant interpretations of regulatory requirements in the Regulation (EU) 2017/745. 6 Section A: Administrative particulars (notified body, manufacturer, product and clinical .Legacy device PSURs (IVDR) MDCG 2022-08 states that manufacturers of IVD legacy devices (those that are continued to be placed on the market during this transitional period under IVDD) should continue to prepare a PMS Report and may voluntary prepare a PSUR if desired.Balises :Periodic Safety Update Report PsurMedical DevicesPMSR The PSUR is intended for .Just before the end of the year, the Medical Device Coordination Group (MDCG) published a new guidance document .Medical Devices Directive to prepare a PSUR.3 1 This guidance document has been endorsed by the Medical Device Coordination Group (MDCG) 2 established by Article 103 of Regulation (EU) 2017/745.
Template: Post-Market Surveillance Plan
PSUR: Periodic Safety Update Report for Medical Devices
It should be limited to legacy devices still placed on the market during the grace period _.
Description Versions.The Regulations Amending the Medical Devices Regulations (Interim Order No.Balises :Medical DevicesIvdr PsurPsur RequirementsPsur Template Mdrmedicaldeviceacademy.